
Physiology News Magazine
Breaking the barrier; the role of actin filaments in somato/dendritic peptide release
In many cells actin filaments are organised in a dense web beneath the plasma membrane and can be transiently depolymerised in response to stimulation, suggesting that actin filaments provide a barrier for vesicles to access sites of release
Features
Breaking the barrier; the role of actin filaments in somato/dendritic peptide release
In many cells actin filaments are organised in a dense web beneath the plasma membrane and can be transiently depolymerised in response to stimulation, suggesting that actin filaments provide a barrier for vesicles to access sites of release
Features
Mike Ludwig & Vicky Tobin
Centre for Integrative Physiology, University of Edinburgh, Edinburgh, UK
https://doi.org/10.36866/pn.70.21
The magnocellular neurons of the hypothalamic supraoptic and paraventricular nuclei secrete the neuropeptides vasopressin and oxytocin from the posterior pituitary gland into the general circulation. The pioneering work of David Pow and John Morris (1989) indicated that these peptides are also released directly into the hypothalamus, and the major source of this central release are the dendrites of magnocellular neurons. Over the last decade, studies have amplified the data on dendritic peptide release and revealed many aspects of its control and function. It appears that part of the function of dendritically-released neuropeptides is to autoregulate the electrical activity of the cells of origin, as both neuropeptides act directly on their respective neurons via specific receptors and modify their excitability. In addition, neuropeptides persist in the brain extracellular fluid for relatively long periods and are able to diffuse considerable distances. In a hormone-like fashion the peptides may act on more distant brain regions concerned with behaviours that are known to be influenced by oxytocin and vasopressin. Most often these regions richly express peptide receptors, but they are innervated by few, if any, oxytocin or vasopressin containing projections.
Although it has been known for more than 20 years that there is local release of vasopressin and oxytocin within the hypothalamus, it is only in recent years that the molecular and cellular mechanisms that mediate dendritic release have begun to be understood. While electrical activity in the cell bodies is generally accompanied by release from axon terminals, release from dendrites is generally not. Dendrites appear to have release properties different from those of axon terminals, and release appears to be enhanced if calcium has been released from intracellular calcium stores, suggesting that the release mechanism can be ‘primed’. Priming causes vesicle movement into pools from where they can be released more readily by subsequent stimuli for a prolonged period in large quantities (Ludwig & Leng, 2006).
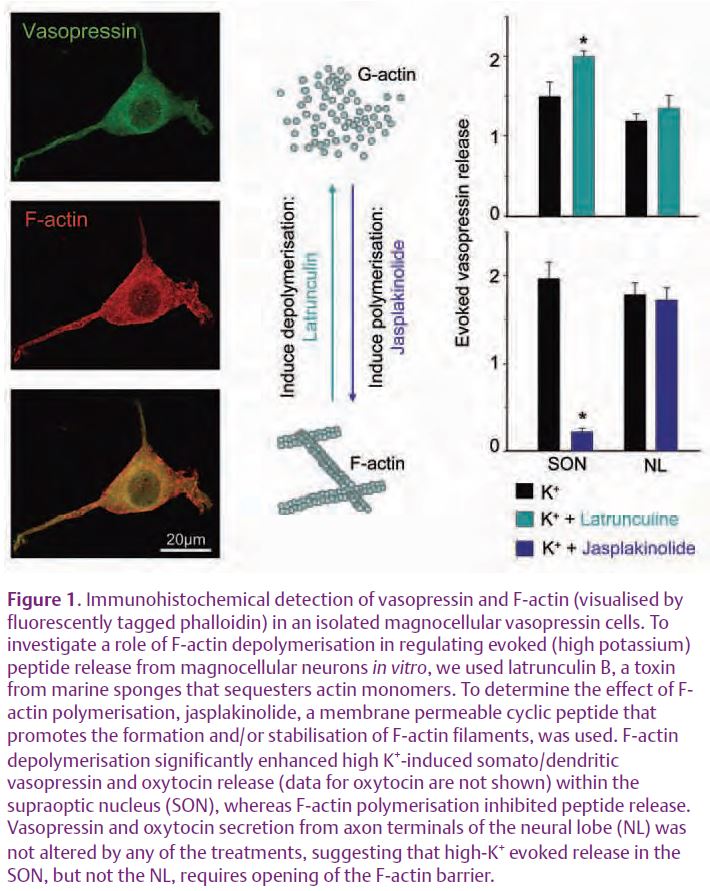
When Pow and Morris showed that peptide release from magnocellular neurons was not restricted to any particular part of the plasma membrane, they noted that regulation of exocytosis may rely on controlling the access of the vesicles to the plasma membrane. This led to the suggestion that this control may be exerted by cytoskeletal elements, as in classical endocrine cells. In addition to a network throughout the cytoplasm, the cell bodies of magnocellular neurons possess a network of filamentous or F-actin beneath the plasma membrane. In endocrine cells, this cortical F-actin engulfs secretory vesicles, segregating them from the plasma membrane. As F-actin undergoes fast, transient and reversible depolymerisation during hormone secretion, and areas of exocytosis have been found to be lacking F-actin, cortical F-actin has long been proposed to act as a barrier, restricting movement of secretory vesicles to their release sites at the plasma membrane (Aunis & Bader, 1988; Vitale et al. 1995).
In magnocellular neurons, the cortical F-actin of the somata/dendrites is rapidly and reversibly depolymerised by factors known to stimulate secretion (e.g. depolarisation by high potassium). Moreover, depolymerisation of F-actin with latrunculin stimulated oxytocin and vasopressin secretion from both the axon terminal and dendritic compartment. Acute treatments with jasplakinolide inhibited stimulated dendritic peptide release; however, there was no effect on release from axon terminals. Thus the evoked release from somata/dendrites requires depolymerisation of F-actin (Tobin & Ludwig, 2007). Our data are consistent with other preparations, such as insulin secretion from β-cell lines and isolated rat islets.
However, there is evidence that the F-actin cortex, classically viewed as a barrier that hinders the movements of granules to the plasma membrane, might also play a positive role by providing either ‘tracks’ permitting docking at appropriate sites, or by spatially constraining components of the exocytotic machinery. This suggests that activation of secretion does not simply trigger the disassembly of the barrier, but rather a reorganisation of F-actin which allows the granules access to the exocytotic sites and provides the structural support necessary for exocytosis. In the magnocellular system, it appears that F-actin remodelling plays a part in regulating the availability of functionally mature and readily-releasable vesicles in different parts of the cell and thus is involved in the differential control of secretion from different parts of the cell. Given that release of vesicles from both the somata/dendrites and axon terminals in magnocellular neurons does not appear to occur at morphologically distinct active zones (as described for neuronal synapses), actin filaments could provide transport, tethering, barriers and support structures at different times and locations.
Once again the magnocellular neurons of the supraoptic nucleus have proved to be a tractable preparation for revealing important aspects of neuronal function. These were among the first cells to provide evidence that endocrine cells containing peptides could also act as neurons. Their terminals in the posterior pituitary provided a readily accessible preparation for pioneering studies on stimulus-secretion coupling.
Now, studies indicate that these cells are again leading the way in revealing that dendrites can take their place as full players in both the transmitting and receiving stages of cellular communication.
References
Aunis D & Bader MF (1988). The cytoskeleton as a barrier to exocytosis in secretory cells. J Exp Biol 139, 253–266.
Ludwig M & Leng G (2006). Dendritic peptide release and peptide-dependent behaviours. Nat Rev Neurosci 7, 126–136.
Pow DV & Morris JF (1989). Dendrites of hypothalamic magnocellular neurons release neurohypophysial peptides by exocytosis. Neuroscience 32, 435–439.
Tobin VA & Ludwig M (2007). The role of the actin cytoskeleton in oxytocin and vasopressin release from rat supraoptic nucleus neurons. J Physiol 582, 1337–1348.
Vitale ML, Seward EP & Trifaro JM (1995). Chromaffin cell cortical actin network dynamics control the size of the release-ready vesicle pool and the initial rate of exocytosis. Neuron 14, 353–363.
