
Physiology News Magazine
Breathing powerfully modulates human vagal and sympathetic motoneurone responsiveness
Fluctuations of vagal and sympathetic motoneurone membrane potentials alter their susceptibility to stimulatory inputs and thereby order the timing of efferent neural outflow from the human central nervous system, writes Dwain Eckberg
Features
Breathing powerfully modulates human vagal and sympathetic motoneurone responsiveness
Fluctuations of vagal and sympathetic motoneurone membrane potentials alter their susceptibility to stimulatory inputs and thereby order the timing of efferent neural outflow from the human central nervous system, writes Dwain Eckberg
Features
Dwain L. Eckberg
Departments of Medicine and Physiology, Hunter Holmes McGuire Department of Veterans’ Affairs Medical Center and Medical College of Virginia at Virginia Commonwealth University, Richmond, Virginia, USA
https://doi.org/10.36866/pn.53.11

In 1733 Stephen Hales, a vicar in the London borough of Richmond upon Thames, published his direct observations on fluctuations of the level of blood in a glass tube inserted into a crural artery of a mare ‘tied down alive on her back’(Hales, 1733):
‘When it was its full height, it would rise and fall at and after each pulse two, three, or four inches, and have there for a time the same vibrations up and down at and after each pulse, as it had when it was at its full height; to which it would arise again, after forty or fifty pulses.’
Study of haemodynamic rhythms continues and, in recent decades, has been extended to those of conscious humans, who can be evaluated safely with noninvasive methods, and whose rhythms can be explicated by elegant, computer-based algorithms. Respiratory-frequency rhythms are of peculiar importance in humans, because human subjects bring to the laboratory a unique capability – they can control their breathing precisely. This article, which is based on a more extensive recent review (Eckberg, 2003), treats human autonomic rhythms at breathing frequencies, as manifestations of ongoing neurophysiological processes.
Breathing orders autonomic neural outflows
Figure 1 illustrates how breathing modulates the intervals between heart beats (the ‘R-R intervals’, whose abrupt changes reflect the ebb and flow of vagal-cardiac nerve activity). In this simple nine minute experiment, the volunteer was asked to breathe at steadily decreasing frequencies, between 15 and three breaths min_1.
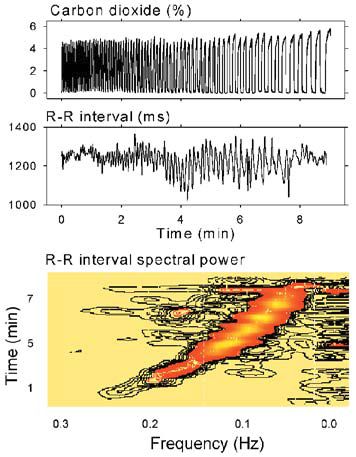
Respiratory excursions of R-R intervals (middle panel) were small at the most rapid breathing rate (left), increased as breathing slowed (centre), and then decreased at the slowest breathing rate (right). The bottom panel is a horizontal section of a sliding fast Fourier transformation moved from the beginning of the period (bottom) to the end (top). R-R interval spectral power importantly tracked breathing frequency (diagonal black-to-orange swath). Low-frequency rhythms, (bottom panel, extreme right), were present throughout the recording, and appeared to be uninfluenced by breathing.

In another experiment (Badra et al. 2001 ), volunteers hyperventilated for two min whilst breathing 100% oxygen, and then held their breaths for as long as they could. Figure 2 shows measurements obtained from one volunteer during uncontrolled breathing and apnoea. The left panels depict horizontal sections through the subject’s sliding R-R interval power spectra, and the right panels depict single power spectra made from the entire three min time series. During uncontrolled breathing, respiratory-frequency R-R interval fluctuations tracked spontaneous breathing frequency fluctuations (left upper panel, right). Spectral power for the entire three min period documented strong periodicity at the breathing frequency, about 0.2 Hz, or one breath every five s (right upper panel, right).
During apnoea, fluctuations at former breathing frequencies were absent (lower panels, right). Therefore, these results suggest that it is breathing itself that is responsible for respiratory-frequency fluctuations of vagal-cardiac nerve activity; they are not driven by some other oscillator which, in turn, modulates respiration.
Breathing gates vagal and sympathetic motoneurone responsiveness
In 1976, Lopes and Palmer advanced the provocative notion that breathing somehow ‘gates’ the responsiveness of vagal-cardiac motoneurones to stimulation. My colleagues and I (Eckberg & Orshan, 1977; Eckberg et al. 1980) followed up on studies conducted earlier in animals and altered baroreceptor input by changing pressure in a neck chamber. When suction is applied to the neck, pressure within the carotid arteries pushes against a vacuum, the carotid arteries and the carotid arterial baroreceptors stretch, vagal-cardiac nerve activity increases, and the heart rate slows. Neck suction is perceived as rising arterial pressure, and neck pressure (which compresses carotid arteries) is perceived as falling pressure.
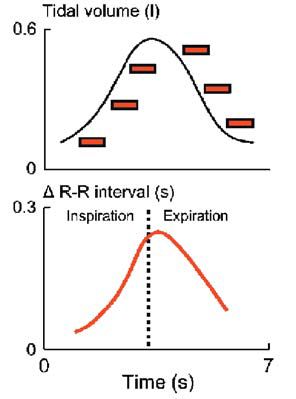
Figure 3 depicts average responses of six healthy young volunteers to brief (less than one s) neck suction, applied at six different times in the breathing cycle. The upper panel shows subjects’ average tidal volume, and the timing of each neck suction pulse. The lower panel shows average R-R interval responses to neck suction, plotted according to the timing of neck suction. The static measurements depicted in the lower panel of Fig. 3 illustrate dynamic neurophysiological changes occurring within the central nervous system during each breath. They show that vagal responsiveness to presumed identical surges of incoming arterial baroreceptor traffic is not uniform over time. Vagal-cardiac motoneurones are minimally responsive when baroreceptor stimuli arrive in early inspiration and late expiration (extreme left and right), and are maximally responsive when baroreceptor stimuli arrive in late inspiration and early expiration (centre).
In a subsequent study (Eckberg et al.1985), we documented similar phasic responsiveness of sympathetic muscle motoneurones to reductions of baroreceptor input (neck pressure), during the breathing cycle. Therefore, responsiveness of both medullary vagal-cardiac and spinal sympathetic-muscle motoneurones varies continuously during quiet breathing in healthy human subjects.
This discussion focuses on how respiration gates autonomic motoneurone responses to very brief changes of baroreceptor input; other research shows that respiration also gates autonomic activity at different steady-state levels of baroreceptor stimulation (Eckberg et al. 1988).
These data introduce a new concept to the issue of respiratory gating: the magnitude of efferent autonomic neural fluctuations secondary to gating is determined by the magnitude of stimulation of motoneurones. Respiratory fluctuations are large when the level of stimulation is large (as with low pressure and sympathetic activity, or high pressure and vagal activity). An obvious corollary of this is that when the level of stimulation is very low, respiratory gating of motoneurones may be absent.
These data do not answer the question: ‘how is respiratory gating of autonomic activity expressed when levels of motoneurone stimulation are extremely high?’ Anrep et al.1936) measured peak minus valley R-R interval fluctuations in spontaneously breathing dogs over a wide range of arterial pressures. They reported that inspiratory-expiratory R-R interval differences vary parabolically over the arterial pressure range: at very high (as well as at very low) arterial pressure levels, respiratory gating of R-R intervals is absent.
We (Eckberg & Orshan, 1977) performed a related experiment in humans. We found (as discussed above) that vagally-mediated R-R interval lengthening provoked by moderate levels of neck suction is greater when stimuli are applied in expiration than inspiration. However, R-R interval lengthening provoked by intense neck suction is equal when stimuli are applied in expiration and inspiration. In another experiment (Cooke et al. 1999), we increased muscle sympathetic nerve activity physiologically, with graded passive upright tilt, and measured the amounts of muscle sympathetic nerve activity occurring during inspiration and expiration. The results indicate that increasing levels of sympathetic motoneurone stimulation lead to decreasing inspiratory -expiratory differences of sympathetic outflow. Therefore, human respiratory gating of both vagal-cardiac and sympathetic-muscle motoneurones is finite, and can be overcome by high levels of motoneurone stimulation.
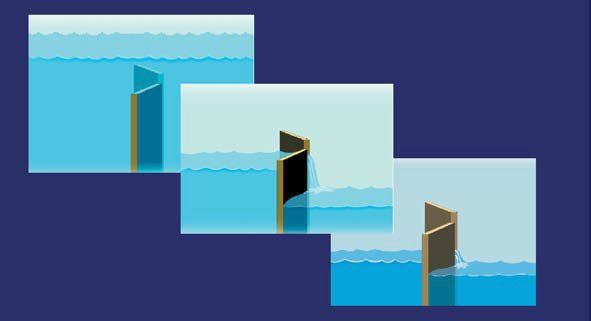
These results speak to the complexity of respiratory gating of autonomic motoneurone firing. The gate is not binary, open or closed; the level of gating is continuously variable (Fig. 3). The gate is not simply shut in inspiration and open in expiration; the gate is open widely in late inspiration, and nearly shut in late expiration. Finally, respiratory gating does not exist independent of the level of the stimulation whose effects are gated; gating is driven by the level of motoneurone stimulation, and can be wholly absent when that level is low or high. This last concept is illustrated schematically in Fig. 4. In this drawing, respiratory gating is represented by a lock in a canal. The respiratory gate opens and closes independent of the level of stimulation (which is represented by the height of the water before the gate). This drawing illustrates the fact that periodic fluctuations of the water level downstream from the gate depend critically on the level of water upstream from the gate. In an elegant study performed in conscious cats, Gilbey and coworkers (1984) explored the electrophysiological basis for respiratory grouping of vagal-cardiac motoneurone firing. They showed that although ambiguous nucleus neurones receive baroreceptor inputs with fixed latencies after arterial pulses, vagalcardiac motoneurone responsiveness depends on other membrane potential fluctuations occurring in synchrony with respiration. The ability of vagal-cardiac motoneurones to fire in response to excitant amino acids fluctuates in parallel with these respiratory fluctuations of vagal-cardiac motoneurone membrane potentials.
Respiratory gating expressions in resting humans
Sinus arrhythmia, the rhythmic heart rate changes that occur during quiet breathing, reflects fluctuating levels of vagus traffic to the sinoatrial node, and is mediated by respiratory gating of vagal- cardiac motoneurone responsiveness to stimulation. We recently documented another expression of respiratory motoneurone gating (Rothlisberger et al. 2003). At rest, systolic pressures and R-R intervals episodically rise and fall together. These parallel changes are regarded as expressions of ongoing baroreflex physiology.
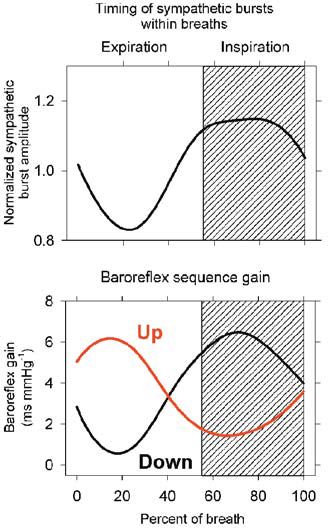
Although such ‘baroreflex sequences’ occur at frequencies lower than breathing frequencies (Badra et al. 2001), we considered that they may, nonetheless, be influenced by breathing (Rothlisberger et al. 2003). Figure 5 shows signal-averaged (on early expiration) muscle sympathetic nerve activity (upper panel) and parallel sequences of increasing (‘up’ sequences) or decreasing (‘down’ sequences) systolic pressures and R-R intervals. The lower panel indicates clearly that respiration modulates the timing of both up and down baroreflex sequences. It is likely that the timing of both up and down baroreflex sequences is determined by respiratory gating of muscle sympathetic nerve activity. Sympathetic bursts occur primarily in expiration and, after a short latency, trigger sequential arterial pressure elevations and baroreflex- mediated R-R interval lengthening. As responses to sympathetic bursts wear off, up sequences are supplanted by down sequences.
Conclusions
In healthy resting human subjects, breathing gates responsiveness of both vagal- cardiac and sympatheticmuscle motoneurones, such that responsiveness is maximal in late inspiration and early expiration, and minimal in early inspiration and late expiration. Fluctuations of efferent autonomic neural outflow secondary to respiratory activity depend critically on the level of stimulation of autonomic motoneurones. At low levels of stimulation (as with high arterial pressure and sympathetic activity, or low arterial pressure and vagal activity), respiratory gating is minimal. Conversely, at high levels of stimulation (as with low arterial pressure and sympathetic activity, or high arterial pressure and vagal activity) respiratory gating may be absent, because respiratory influences have been overcome. At usual arterial pressures, respiratory activity orders R-R interval fluctuations (respiratory sinus arrhythmia), the timing of muscle sympathetic bursts, and thereby, the timing of spontaneous (vagal) baroreflex sequences. It may be that the greatest significance of respiratory gating of autonomic motoneurone responsiveness lies in its utility as a tool to understand otherwise obscure human neurophysiological mechanisms.
Acknowledgments
The research described in this review was supported by longstanding grants and contracts from the National Institutes of Health, the Department of Veterans’ Affairs, and the National Aeronautics and Space Administration.
References
Anrep GV, Pascual W & Rossler R (1936). Respiratory variations of the heart rate. 1- The reflex mechanism of the respiratory arrhythmia. Proc Roy Soc Lond Ser B -BioI Sci 1198,191-217.
Badra LJ, Cooke WH, Hoag JB, Crossman M, Kuusela TA, Tahvanainen KUO & Eckberg DL (2001). Respiratory modulation of human autonomic rhythms. Am J Physiol 280, H2674-H2688.
Cooke WH, Hoag JB, Crossman M, Kuusela TA, Tahvanainen KUO & Eckberg DL (1999). Human responses to upright tilt: a window on central autonomic integration. J PhysioI 517, 617-628.
Eckberg DL (2003). The human respiratory gate. J PhysioI 548, 339352.
Eckberg DL, Rea RF, Andersson OK, Hedner T, Pernow J, Lundberg JM & Wallin BG (1988). Baroreflex modulation of sympathetic activity and sympathetic neurotransmitters in humans. Acta Physiol Scand 133, 221-231.
Eckberg DL, Kifle YT & Roberts VL (1980). Phase relationship between normal human respiration and baroreflex responsiveness. J PhysioI 304, 489-502.
Eckberg DL, Nerhed C & Wallin BG (1985). Respiratory modulation of muscle sympathetic and vagal cardiac outflow in man. J PhysioI 365, 181-196.
Eckberg DL & Orshan CR (1977). Respiratory and baroreceptor reflex interactions in man. J Clin Invest 59, 780-785.
Gilbey MP, Jordan D, Richter DW & Spyer KM (1984). Synaptic mechanisms involved in the inspiratory modulation of vagal cardioinhibitory neurones in the cat. J Physiol 356, 65-78.
Hales S (1733). Statical Essays: Containing Haemastaticks; or, An Account of some Hydraulick and Hydrostatical Experiments made on the Blood and Blood- Vessels of Animals. W Innys, R Man by & T Woodward, London.
ILopes OU & Palmer JF (1976). Proposed respiratory ‘gating’ mechanism for cardiac slowing. Nature 264,454-456.
Rothlisberger BW, Badra LJ, Hoag JB, Cooke WH, Kuusela TA, Tahvanainen KUO & Eckberg DL (2003). Spontaneous ‘baroreflex sequences’ occur as deterministic functions of breathing phase. Clin Physiol Funct Imag (In Press).
