
Physiology News Magazine
Ca2+ phase waves emerge
Biology continues to astonish even in well-studied areas. The discovery of long-range Ca2+ phase waves presents one such example. Dirk van Helden and Mohammad Imtiaz explain
Features
Ca2+ phase waves emerge
Biology continues to astonish even in well-studied areas. The discovery of long-range Ca2+ phase waves presents one such example. Dirk van Helden and Mohammad Imtiaz explain
Features
Dirk F. van Helden & Mohammad S. Imtiaz
The Neuroscience Group, School of Biomedical Sciences, University of Newcastle, NSW, Australia
https://doi.org/10.36866/pn.52.7

Lymphatic pacemaking
Evidence that Ca2+ phase waves can underlie both pacemaking and signal propagation has derived from studies on smooth muscle. My (i.e. DvH’s) interest in pacemaking first arose during studies investigating the properties of spontaneous transient depolarizations in mesenteric veins. These events proved of particular interest as they were determined to be generated by spontaneous Ca2+ release events, each such event generating a spontaneous transient inward current (STIC) (van Helden, 1991) (Fig. 1). As such they presented a corollary to reports of spontaneous transient outward currents (STOCs) but now being excitatory. Adjacent to these vessels were lymphatic vessels that often exhibited spontaneous contractions. These vessels are divided into multiple chambers by unidirectional valves with each chamber acting as a ‘primitive heart’ to pump lymph. At this time (1986-89), there was little knowledge about the pacemaker mechanism in these ‘hearts’ and it was generally assumed that pacemaking arose through a cardiaclike pacemaker model involving cyclical activation of voltagedependent channels in the cell plasmalemma. The temptation caused by observing the ‘hearts’ pumping away was too much and soon my microelectrodes strayed from the venous preparations to recording from the lymphatic smooth muscle.
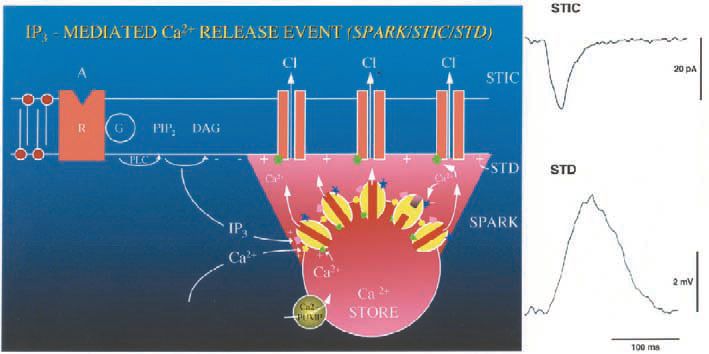
Significantly these vessels, like the arteriolar preparations first prepared by David Hirst and Tim Neild and subsequently the small veins I had been working on, could be cut into electrically short segments. This meant that voltages generated by spontaneous or injected currents could be recorded with little decrement across the syncytium of smooth muscle cells. Indeed, in the mesenteric lymphatic preparation, some of the very short ‘hearts’ operated as independent electrically short preparations due to the smooth muscle being discontinuous in the region of the valves. Intracellular recordings made from electrically short lymphatic preparations led to the first major surprise, as action potentials were found to be generated by spontaneous transient depolarizations with properties analogous to those observed in the venous preparations (van Helden, 1993). Thus Ca2+ stores appear to control electrical pacemaking in these preparations.
The problem of store synchronicity
These findings, while most interesting, left one rather major perplexing problem. This was the problem of how stores could achieve sufficient synchronicity to drive large lymphatic ‘hearts’. The much larger smooth muscle syncytium in such chambers exhibits very low input resistance and would require large currents to drive it, necessitating highly co-ordinated store release. How might stores achieve such synchronicity? The vein studies had shown that STICs show a broad range of amplitudes and could be very large, suggesting that there was substantial synchronisation of store release within and possibly across cells. Evidence for synchronisation of IP3R-mediated Ca2+ release was also provided by the observation that a summation of these events could act as the lymphatic pacemaker potential, triggering the generation of lymphatic action potentials.
Synchronicity through diffusion-based Ca2+ waves still insufficient
A possible solution to this mystery, was provided by the reports of Ca2+ waves where release from one store activates release of the adjacent stores. This occurs through calciuminduced calcium release (CICR) from either ryanodine receptors (RyRs) or inositol 1,4,5-trisphosphate receptors (IP3Rs) present in the
sarcoplasmic/endoplasmic reticulum of cells. Thus group activation of stores could occur by this mechanism which, in the case of lymphatics, would occur by sequential activation of IP3Rs. However, there is a caveat even with this mechanism as Ca2+ waves have been reported to conduct relatively slowly (typically 0.002 – 0.1 mm/s) and would activate relatively few cells given the smooth muscle cell length is the order of 0.1 mm and Ca2+ release events and resultant STICs are brief (e.g. < 0.3 s). As such, even this mechanism is likely to be severely limited in its ability to induce sufficient current to drive guinea-pig lymphatic chambers to threshold, let alone those in the lymphatics of larger animals. Therefore, the question of how stores synchronise had not been resolved.
Slow waves also require massive store synchronicity
The pathway through this impasse came during a brief sabbatical visit to the laboratory of Hikaru Suzuki at Nagoya City University in the beginning of 1995. Here, I was introduced to recording slow waves in a visceral smooth muscle preparation and to the preparation of electrically tractable single bundles of this preparation, the latter provided by the much appreciated help of Takeo Itoh. While long studied, the origin of these waves was still unknown. A surprising similarity in the appearance of this activity to lymphatic pacemaker activity led to pharmacological experiments investigating a role for Ca2+ stores. Importantly, the results paralleled those of the lymphatics indicating that stores were driving pacemaking. This same approach was also applied to slow waves in the guinea-pig urethra resulting in the same outcome that stores were driving the slow waves (Hashitani et al. 1996). A key publication from Jan Huizinga’s laboratory on the canine colon (Liu et al. 1995) arrived at the same conclusion, namely that Ca2+ stores were pacing slow waves.
Importantly, these findings were constrained by the same impasse. How could stores achieve sufficient synchronicity to pace the regenerative slow waves? Furthermore, in visceral smooth muscle there are additional complexities. First, the pacemaker cells are proposed to be a different type of cell termed Interstitial Cells of Cajal (ICCs), with one type present in specific networks in the myenteric plexus (ICC-MP) and another present intramuscularly (ICC-IM). Second, and most importantly, it was not known how the signal propagated across the vast number of cells to, in many cases, produce near synchronous contractions (e.g. circumferential contractions of the stomach including those of large animals are near synchronous providing optimum ‘squeezing’ of stomach contents). This does not occur by voltage-dependent channels commonly associated with action potentials, as slow waves occur and propagate in the presence of TTX or L-type Ca2+ channel blockade.
Synchronicity through coupled oscillators
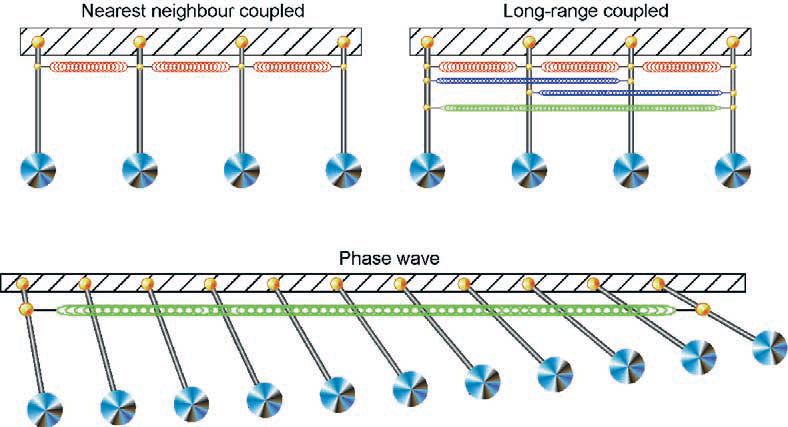
A major advance to explaining the requirement for massive store synchronicity came when back in my own laboratory. There was an ongoing controversy of how slow waves propagate, with a cardiac type model (i.e. action potential-based propagation) contrasted against a long held alternative model, based on the concept of synchronisation of coupled oscillators. Coupled oscillator-based interactions were first described for an array of pendulums that, when linked (e.g. by springs) and randomly activated, entrain their activity over time. The result is a phase wave in which each oscillator has the same frequency of oscillation but with a spatial variation in phase that depends on the strength of the coupling between the oscillators. It is these principles that led to coupled oscillator-based models being applied to biological rhythms, as first applied to model heartbeats (van der Pol & van der Mark, 1926) and subsequently to interpret gastrointestinal slow waves. While these models were mathematically based with no understanding of the underlying biological oscillators, they provided great interest and various research groups have continued to argue strongly for such mechanisms (e.g. see Daniel et al. 1994). This coupled oscillator model provided the next ‘brick in the road’ to understanding store synchronicity.
Diffusion-linked Ca2+ stores interact as weak coupled oscillators
Ca2+ stores are oscillators and are clearly coupled by at least one factor (i.e. the diffusion of Ca2+) within and presumably across the cellular syncytium. We predicted coupling to occur through active stores undergoing oscillatory Ca2+ release advancing or retarding the cycle of adjacent stores by CICR until entrainment was achieved. It was time to undertake simulations of these interactions as made with Mike Sculley and subsequently with my graduate student Mohammad Imtiaz. To do this we used existing models used for modelling sequentially conducting Ca2+ waves involving a one-dimensional array of IP3Roperated stores capable of oscillatory Ca2+ release as stimulated by IP3 and/or Ca2+. The primary difference was that we applied the stimulus globally over the tissue. The result was weak coupled oscillator-based synchronisation. An important paper appeared during this period, using the same type of simulation showing that Ca2+ waves in astrocytes could be explained as arising through Ca2+ stores interacting as coupled oscillators (Roth et al. 1995). In their case the coupled oscillator-based interactions, while weak, could explain the measured ‘propagation’ rates of the Ca2+ waves, which in their tissues exhibited ‘apparent conduction velocities’ (‘CVs’) in the range 5-60 um/s dependent on the level of agonist stimulation and hence intracellular [IP3]. Therefore, we were left with the conclusion that Ca2+ stores could interact as coupled oscillators, but such coupling was weak. This mechanism could not explain slow wave initiation due to the weak synchrony. Secondly, it could not explain slow wave propagation, with slow waves propagating with ‘CVs’ orders of magnitude greater.
Membrane voltage – the ‘missing link’
The next step forward came through the outcomes of experiments being undertaken in the laboratory using finely dissected ‘single bundle’ strips of gastric pyloric smooth muscle (van Helden et al. 2000). Here, we presented evidence that slow waves were composed of a pacemaker and regenerative component, with both components generated by release from IP3R-operated stores. Thus while the regenerative component of the slow wave outwardly resembles an action potential, it is not generated by voltage-dependent channels in the cell plasmalemma but by regenerative release from Ca2+ stores. Significantly, there is voltagedependent feedback on IP3Rmediated Ca2+ release, with depolarization enhancing and hyperpolarization decreasing release. This may result through voltagedependent production of IP3 but the same outcome would arise if the depolarization acted by an intermediate step, such as enhancing [Ca2+]i, with this intermediate then enhancing production of IP3 (Fig. 2). Similar findings were also reported in strips of gastric antrum (Suzuki & Hirst, 1999).
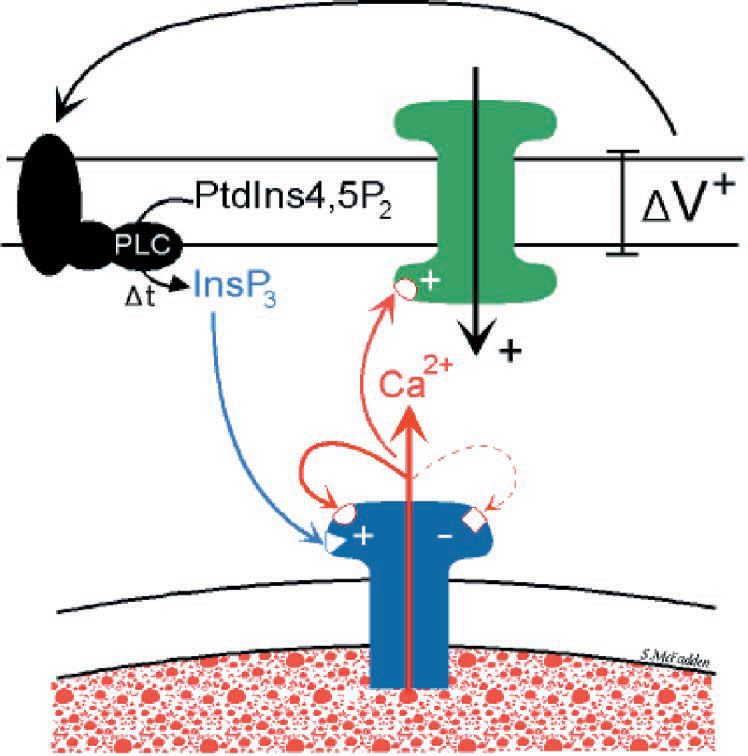
The link between membrane potential and store Ca2+ release provided the key to solving the question of how stores could synchronise on a massive scale. Now stores are not simply linked by the diffusion of store activators alone but also by membrane voltage in its capacity to induce IP3R-mediated Ca2+ release. Thus while the small effective diffusion range for Ca2+ of < 10 µm provides weak coupling between stores, coupling by membrane voltage is much more effective. This is so because membrane voltage and associated current flow has orders of magnitude more ‘reach’ than diffusion with electrical length constants typically in the range of 1–4 mm. The result is that active stores can now synchronise on a grand scale through long-range parallel interactions. It is this voltage link which underpins the emergence of long-range Ca2+ phase waves.
Store synchronicity through long-range Ca2+ phase waves
Our recent paper and simulation (van Helden & Imtiaz, 2003) presents experimental and model-based evidence for the existence of these Ca2+ phase waves. The findings were again made using fine ‘single bundle’ strips of guinea-pig pyloric smooth muscle but now mostly using electrically long tissue strips of length up to 10 mm. Our view of how Ca2+ phase waves emerge in a previously quiescent tissue is as follows. Increasing stimulation of stores (e.g. agonist-induced increases in [IP3]i) across the cellular syncytium first activates the most sensitive subplasmalemmal IP3R-operated stores, the Ca2+ release events causing local inward current flow across the plasmalemma. Initially these act independently but as the level of stimulation increases local interactions increase to generate larger coordinated events underlying measurable spontaneous transient depolarizations. With further stimulation the number of these events increases across the cellular syncytium leading to more global synchronisation and resultant coordinated pacemaker Ca2+ release and associated pacemaker depolarization. As spatial synchronisation is imperfect, there is a phase delay between Ca2+ release across the array of stores and the pacemaker Ca2+ release takes the form of a Ca2+ phase wave. This is presented in the schematic of Fig. 3, which for simplicity uses a pendulum analogy. Further stimulation and associated recruitment of stores, by the pacemaker Ca2+ release together with the associated depolarization, causes the sub-plasmalemmal [Ca2+] and/or [IP3] to reach threshold for regenerative activation of the large population of less sensitive stores. This results in regenerative Ca2+ release and associated slow wave potential. The Ca2+ phase waves are now much larger reflecting a pacemaker and a regenerative component with the average cycle time of the entrained stores determining the frequency and the phase delay the ‘CV’ of the resultant slow waves.
Part of the evidence for Ca2+ phase waves was based on a type of experiment made by A.L. Hodgkin in 1939. He found that alteration of the conduction pathway in a central region of a nerve axon modulated the action potential CV and in one case action potential conduction was interrupted dependent on the effectiveness of conduction in the central region. This latter observation is entirely consistent with the action potential conducting sequentially. In contrast, an analogous experiment performed on a strip exhibiting slow waves made by chemically interrupting the connectivity between cells near the middle of the strip did not stop transmission. Rather, the slow waves persisted at the two strip ends but now showed no phase correspondence with each other. This indicates that interactions are occurring by coupled oscillator-based mechanisms. A role of stores and the importance of electrical coupling between stores were indicated by central application of an inhibitor that blocks store release but does not interrupt cellular connectivity. This produced the same result but required a much wider central region of inhibition commensurate with the hypothesis that stores were primarily coupled by membrane voltage and not by diffusion of Ca2+. In summary, the findings indicate that stores interact as coupled oscillators and that membrane potential is a key linking factor between the stores.
Simulations made by modelling the tissue as an array of IP3R-operated stores with a Gaussian distribution of sensitivities mimic all the experimental findings. These simulations again confirm the fundamental importance of linking membrane depolarization to IP3Rmediated store Ca2+ release. Significantly, the tissue can operate effectively independent of tissue size due to the distributed nature of the pacemaker, with each cell likely to drive its own regenerative response.
The role of specific ‘pacemaker cells’ is also readily accounted for, these corresponding to cells that more readily undergo oscillatory Ca2+ release with their oscillatory cycle in turn entraining cells with less sensitive Ca2+ stores. Importantly, the proposed feedback between depolarization and production of IP3 as used in the modelling studies indicates the co-existence of IP3phase waves. These are predicted to operate hand in hand with the Ca2+ phase waves.
Significantly, very high levels of agonist stimulation can cause desynchronisation and failure of slow waves. Our simulations suggest that this occurs because the frequency of store oscillations, which is known to increase with agonist concentration , becomes too high so that existing delays in the feedback between membrane voltage and store release become significant with resultant weakening in store coupling.
Lymphatic vasomotion
The same hypothesis can also be applied to lymphatics, as we together with Jun Zhao have recently communicated. The key to store synchronicity is again feedback between membrane depolarization. However, now the link is primarily due to depolarization induced opening of L-type Ca2+ channels and resultant calcium-induced calcium release from the stores. Ca2+ stores within the smooth muscle syncytium are now strongly coupled by membrane voltage and synchronise to produce pacemaker potentials that trigger action potentials and constrict the lymphatic ‘hearts’.
Such constrictions are a type of vasomotion and in this regard it is interesting to note a recent hypothesis for vasomotion (Peng et al. 2001). Here initial uncoordinated Ca2+ release is considered to synchronise when ‘sufficient number of cells become active at the same moment’. The resultant inward current now overcomes the current sink in the preparation and depolarizes all cells to open L-Ca2+ channels, the resultant Ca2+ influx activating Ca2+ release across the cells. Significantly, Ca2+ phase waves provide a means by which biology ensures that sufficient number of cells do become active at the same moment. Consistent with this, there is direct proof in the literature that lymphatic vessels constrict through a coupled oscillator-based process. This derives from studies on bovine lymphatics (McHale & Meharg, 1992). Here central interference with pacemaking by either reduction in temperature or application of heptanol (10 mM), an agent likely to block intercellular connectivity caused the near synchronous contractions along the vessel to decouple.
Voltage-accelerated Ca2+ waves
A point made in our study (van Helden & Imtiaz, 2003) is that local stimulation of Ca2+ release could lead to production of accelerated sequentially conducting Ca2+ waves. This is considered to occur in the gastrointestinal smooth muscle through the voltage-feedback producing enhancement in IP3Rmediated Ca2+ release, this process then continuing in sequence along the tissue. In the likely event that the feedback between membrane potential and IP3R-mediated Ca2+ release is due to increased [IP3], then there will be both a sequential voltage-accelerated IP3 wave and resultant Ca2+ wave. However, while such rapidly conducting sequential waves are likely to be very important, the normal modus operandi of the rhythmically active tissues of our studies (i.e. pyloric and lymphatic smooth muscle tissues) is through stores interacting as coupled oscillators.
Future directions
Ca2+ and/or IP3 phase waves may emerge to generate cellular rhythms when cells:
1. exhibit oscillatory IP3R- or RyRmediated store Ca2+ release;
2. depolarize in response to store Ca2+ release;
3. demonstrate increased store Ca2+ release upon membrane depolarization and
4. are interlinked through gap junctions or other pathways.
Physics tells us that arrays of oscillators that are strongly coupled entrain when sufficiently stimulated. Therefore Ca2+ stores in such cell systems should interact as strongly coupled oscillators, exhibiting emergent local near synchronous oscillations and long-range Ca2+ phase waves.
In such systems pacemaker frequency would be determined by the cycle time of the entrained stores and ‘CV’ by the synchronicity of the phase waves. As for the former, we note that Ca2+ phase wave-based pacemaking can theoretically exhibit an enormous range of frequencies (e.g. <0.1 to >10Hz), consistent with reported store cycle times. We also note that the ‘CV’ may also show a large range depending on factors such as the connectivity of cells and the strength of the positive feedback between membrane depolarization and store Ca2+ release.
Ca2+ phase wave-based pacemaking as presented here is sufficiently novel to allow some speculation. First, we predict it to underlie many cellular rhythms including specific brain rhythms, the brain being replete with both IP3Rs and RyRs. Second, it seems possible that it will also have a role in heart pacemaking and conduction. We make this prediction based on growing evidence for a role of stores, as an important source of pacemaker current and through the strong linkage between membrane potential (i.e. voltage-dependent Ca2+ entry) and store Ca2+ release. Perhaps this store-based mechanism of pacemaking, as first observed in lymphatic ‘hearts’, may prove to be less ‘primitive’ than previously thought.
References
Daniel EE, Bardakjian BL, Huizinga JD & Diamant NE (1994). Relaxation oscillator and core conductor models are needed for understanding of GI electrical activities. Am J Physiol 266, G339349.
Hashitani H, Van Helden DF & Suzuki H (1996). Properties of spontaneous depolarizations in circular smooth muscle cells of rabbit urethra. Brit J Pharmacol 118, 1627-1632.
IJLiu LW, Thuneberg L & Huizinga JD (1995). Cyclopiazonic acid, inhibiting the endoplasmic reticulum calcium pump, reduces the canine colonic pacemaker frequency. J Pharmacol Exp Ther 275, 1058-1068.
McHale NG & Meharg MK (1992). Co-ordination of pumping in isolated bovine lymphatic vessels. J Physiol 450, 503-512.
Peng H, Matchkov V, Ivarsen A, Aalkjaer C & Nilsson H (2001). Hypothesis for the initiation of vasomotion. Circ Res 88, 810-815..
Roth BJ, Yagodin SV, Holtzclaw L & Russell JT (1995). A mathematical model of agonist-induced propagation of calcium waves in astrocytes. Cell Calcium 17, 53-64.
Suzuki H & Hirst GD (1999). Regenerative potentials evoked in circular smooth muscle of the antral region of guinea-pig stomach. J Physiol 517, 563-573.
van der Pol B & van der Mark J (1926). The heartbeat considered as a relaxation oscillation, and an electrical model of the heart. Phil Magnus Suppl. 6, 763-775.
van Helden DF (1991). Spontaneous and noradrenaline-induced transient depolarizations in the smooth muscle of guinea-pig mesenteric vein. J Physiol 437, 511-541.
van Helden DF (1993). Pacemaker potentials in lymphatic smooth muscle of the guinea-pig mesentery. J Physiol 471, 465-479.
van Helden DF & Imtiaz MS (2003). Ca2+ phase waves: a basis for cellular pacemaking and long-range synchronicity in the guinea-pig gastric pylorus. J. Physiol. 548.1, 271-296.
van Helden DF, Imtiaz MS, Nurgaliyeva K, von der Weid P & Dosen PJ (2000). Role of calcium stores and membrane voltage in the generation of slow wave action potentials in guinea-pig gastric pylorus. J Physiol 524, 245-265.
