Physiology News Magazine
Calcium: it’s not just for bones!
Calcium ions in the human body have diverse physiological functions. Recently, extracellular calcium ions and a G protein-coupled, calcium-sensing receptor have been shown to play a role in developmental lung physiology. By increasing our knowledge of the lung development process, we may be able to pursue novel treatments that could improve outcomes for infants born prematurely or born with developmental lung disorders
Features
Calcium: it’s not just for bones!
Calcium ions in the human body have diverse physiological functions. Recently, extracellular calcium ions and a G protein-coupled, calcium-sensing receptor have been shown to play a role in developmental lung physiology. By increasing our knowledge of the lung development process, we may be able to pursue novel treatments that could improve outcomes for infants born prematurely or born with developmental lung disorders
Features
Brenda A Finney (1), William J Wilkinson (1), Paul J Kemp (1), and Daniela Riccardi (1,2)
1: School of Biosciences and 2: Cardiff Institute of Tissue Engineering and Repair (CITER), Cardiff University, UK
https://doi.org/10.36866/pn.75.25

Calcium is an ion with diverse roles
When talking with friends and family, if you say that you work with calcium ions, most automatically assume that you also work with bones. Correct this assumption to tell them that you are looking at the effects of Ca2+ on lung development and their next question becomes, ‘There is calcium in the lungs?’ The answer to this question is, of course, ‘There is calcium throughout the body’. There are many processes, from the formation of bones to the contraction of muscles and beyond, that are dependent upon Ca2+.
Aside from its direct actions, Ca2+ can act as a signalling molecule through a G protein-coupled receptor called the calcium-sensing receptor (CaR). Indeed, our bodies maintain a constant systemic free-ionised extracellular Ca2+ concentration ([Ca2+]o) by integrating the actions of several tissues including the parathyroid gland, intestine, kidney and bone. In the adult, parathyroid CaR keeps systemic [Ca2+]o at approximately 1.2 mM. In the fetus, [Ca2+]o is higher than in the mother, as reported in mice, sheep and humans. The fetal CaR is involved in the maintenance of this relative hypercalcaemia, which lasts throughout gestation, and is resolved to normocalcaemia within 24 hours after birth (Kovacs et al. 1998). While late in gestation the majority of Ca2+ obtained by the fetus is deposited in the developing skeleton, other physiological effects of this relative hypercalcaemia during development, are only recently emerging. In our study (Finney et al. 2008), we have shown that Ca2+o is an important, extrinsic factor which regulates several aspects of the intrinsic developmental programme of the lung.
Calcium and lung development

In order to meet its primary function after birth – gas exchange – the developing lung must undergo a process of branching, growth and differentiation. These processes are accompanied by airway peristalsis, stereotypic branching morphogenesis and chloride secretion into a fluid-filled lumen (Fig. 1A). Together, these events ensure that there is a correct number of optimally distended terminal airway buds which contain the requisite cellular components so that the emerging fetus can efficiently take its first breath.
In embryonic rat lungs, the airway smooth muscle (ASM) lining the basal side of the developing airway epithelium, exhibits spontaneous peristaltic movements which are dependent on an intracellular Ca2+ (Ca2+i) wave originating from a pacemaker region in the right proximal lung. This phenomenon, which is dependent upon Ca2+o influx, can be correlated with fetal lung growth, that is, an increase in lung growth causes an increase in peristalsis, and a decrease in peristalsis decreases lung growth (Jesudason et al. 2005). Ca2+i imaging has revealed that regenerative, spontaneous waves (Fig. 1B, top trace) travel via gap junctions through the ASM. Nominally Ca2+o-free solution applied to cultured lung explants causes both the Ca2+i signalling and peristalsis to cease completely (Fig. 1B, bottom trace), but these processes restart when Ca2+o is reapplied (Featherstone et al. 2005). Therefore, both Ca2+o and Ca2+i signalling are vital components of ASM peristalsis and the growth of the developing lung. While these studies show that both the presence of Ca2+o and Ca2+i signalling are necessary, there is no information regarding how the chronically elevated [Ca2+]o of the fetus may effect lung growth and development.
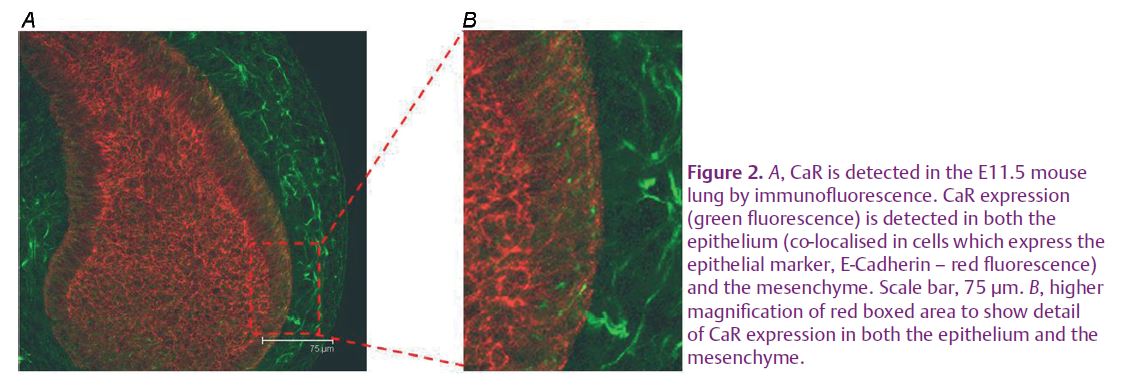
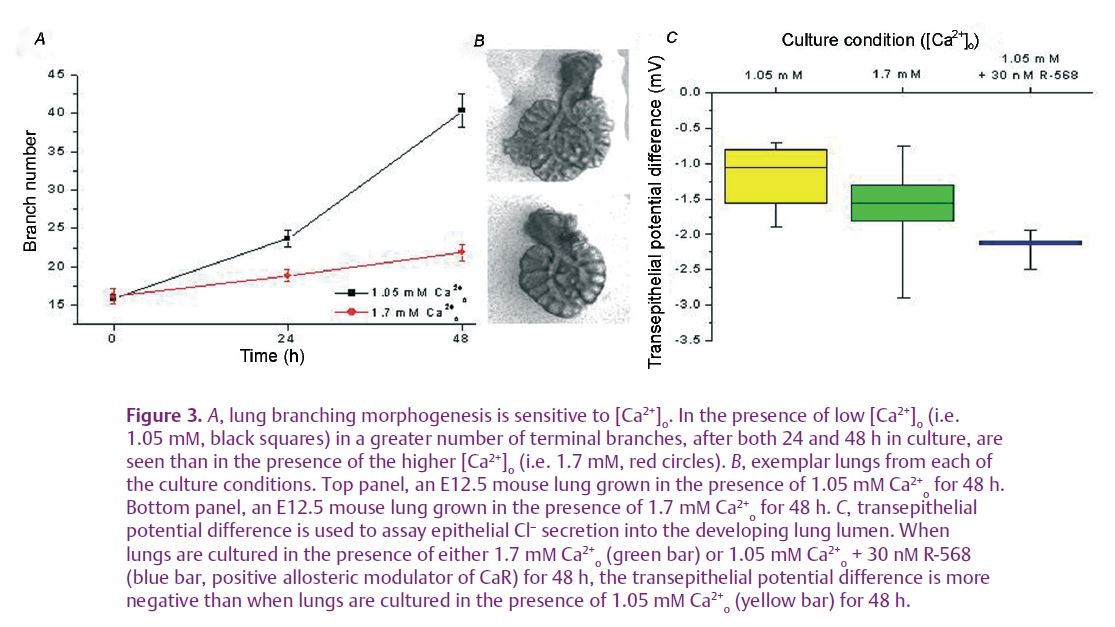
Recently, we have found that the CaR is expressed and active during mouse lung development (see Finney et al. 2008 and Figs 2 and 3). This receptor exhibits a developmentally regulated pattern of expression which peaks at the time corresponding to lung branching morphogenesis. Using serum-free, chemically defined medium containing 1.7 mM Ca2+o (corresponding to [Ca2+] o in late gestation, fetal plasma), embryonic day 12.5 mouse lungs cultured for 48 hours undergo significantly less branching morphogenesis than that achieved by explants grown in the presence of adult [Ca2+] o (1.05–1.2 mM, Fig. 3A and B). Activation of the CaR with a specific positive allosteric modulator of the receptor, R-568, mimics the effects of the higher [Ca2+]o in that it too restricts the rate of branching morphogenesis. This CaR-dependent control of branching occurs through phospholipase C and phosphotidylinositol 3-kinase signalling pathways. Additionally, secretion of chloride into the lung lumen, a vital part of forming the liquid template around which the lung branches grow, is also affected by Ca2+o and CaR activation. Thus, chloride secretion, measured as transepithelial potential difference, is higher in the presence of 1.7 mM Ca2+o than it is in 1.05 mM Ca2+o, and it is increased even further upon specific activation of the CaR by R-568. (Fig. 3C).These data are the first demonstration that Ca2+o, acting through the CaR, have long-term effects on lung development. These data imply that [Ca2+] and CaR-dependent signalling are involved in the formation and expansion of peripheral airways. Our initial interpretation of these data is that CaR activation at fetal [Ca2+] o (approximately 1.7 mM) acts as a physiological brake on branching morphogenesis while enhancing chloride secretion. The physiological significance of these opposing effects may be to ensure matching of airway branching with expansion to optimise the gas transfer interface.
The importance of getting lung development right
Perturbations of the developmental programme which impinge upon this important developmental balance can result in decreased functionality of the postnatal lung. Disruption of lung development can be caused by malformations of the chest cavity, exposure to toxins, inappropriate gene expression, fetal growth restrictions or premature birth. Premature birth is a significant problem which inherently carries with it the risks of inadequate lung development. The inadequacies of the premature lung are exacerbated by instances of respiratory disease such as respiratory distress syndrome (RDS) or bronchopulmonary dysplasia (BPD; Warburton & Bellusci, 2004).
Indeed, RDS is a major cause of morbidity and mortality in premature infants. In the UK for the year 2001, 58% of newborn deaths occurring before the infant had reached 28 days old were due to RDS or an extremely low weight at birth (National Office of Statistics, http://www.statistics.gov.uk/pdfdir/chi0303.pdf). There is also evidence to suggest that children who are born prematurely and suffer RDS or BPD have a higher incidence of respiratory illness, as well as decreased respiratory flow and diffusion capacity later in life (Moss, 2006).
Along with the physical symptoms detailed above, there are significant socio-economic impacts of prematurity which can last for the lifetime of the affected individuals. In order to reduce the number of deaths and improve the outcomes for these individuals, we must first understand normal developmental lung physiology. By incorporating our new data regarding Ca2+ and CaR signalling into the established paradigms of developmental lung physiology, we may be one step closer to ameliorating the suffering of many infants and adults by the development of interventions that could promote the formation of healthy lungs.
References
Featherstone NC, Jesudason EC, Connell MG, Fernig DG, Wray S, Losty PD & Burdyga TV (2005). Spontaneous propagating calcium waves underpin airway peristalsis in embryonic rat lung. Am J Respir Cell Mol Biol 33, 153–160.
Finney BA, del Moral PM, Wilkinson WJ, Cayzac S, Cole M, Warburton D, Kemp PJ & Riccardi D (2008). Regulation of mouse lung development by the extracellular calcium-sensing receptor, CaR. J Physiol 586, 6007–6019.
Jesudason EC, Smith NP, Connell MG, Spiller DG, White MRH, Fernig DG & Losty PD (2005). Developing rat lung has a sided pacemaker region for morphogenesis-related airway peristalsis. Am J Respir Cell Mol Biol 32, 118–127.
Kovacs CS, Ho-Pao CL, Hunzelman JL, Lanske B, Fox J, Seidman JG, Seidman CE & Kronenberg HM (1998). Regulation of murine fetal–placental calcium metabolism by the calcium-sensing receptor. J Clin Invest 101, 2812–2820.
Moss TMJ (2006). Respiratory consequences of preterm birth. Clin Exp Pharmacol Physiol 33, 280–284.