
Physiology News Magazine
Calcium signalizing in developing mouse skeletal muscle fibres
Mice are born immobile and develop the ability to move over the first 2-3 weeks of life. Here Carlo Caputo and co-workers explore the underlying processes in skeletal muscle
Features
Calcium signalizing in developing mouse skeletal muscle fibres
Mice are born immobile and develop the ability to move over the first 2-3 weeks of life. Here Carlo Caputo and co-workers explore the underlying processes in skeletal muscle
Features
Joana Capote, Pura Bolaños, & Carlo Caputo
Centro de Biofisica y Bioquimica, Instituto Venezolano de Investigaciones Cientificas IVIC, Caracas, Venezuela
https://doi.org/10.36866/pn.62.33

Recently-born mice lack the capacity for movement, which is slowly acquired during the 2-3 weeks following birth. During this period, important changes take place at level of the molecular entities, schematically listed in Fig. 1, responsible for the series of events that start with membrane excitation and lead to massive calcium release and contractile activation in skeletal muscle fibres (Capote et al. 2005).

The records in Fig. 2 compare Ca2+ transients obtained with enzymatically dissociated flexor digitorum brevis muscle fibres from 7 and 42 day old mice, respectively. In adult animals the time course of Ca2+ transient is characterized by a fast rising phase, completed in about 1.5 ms, followed by a bi-exponential decay phase, with time constants of about 2 and 16 ms respectively. In 7 day old mice, the magnitude of Ca2+ transients is about one third while the raising phase of the transients is double that in adult fibres, while the bi-exponential decay phase is much slower, with time constants of 5 and 105 ms respectively.
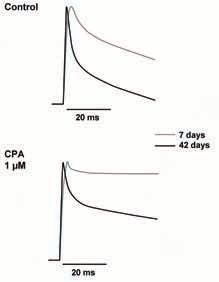
Ca2+ currents in recently- born mice (Beam & Knudson, 1988) appear to contribute more to Ca2+ signalizing than in adult animals; thus in the absence of external Ca2+ the transient amplitude is diminished by 25% in the former and by 11% in the latter.
In principle the prolonged duration of the Ca2+ transients in young animals could be due to prolonged duration of action potentials caused by incomplete development of the K+ delayed rectifier and by the presence of Ca2+ currents. However, optical measurements with the potentiometric dye Di-8-ANEPPS indicate that the duration of action potential is practically the same in young and adult fibres, precluding this possibility.
Alternatively, the slow decay of Ca2+ transients in young fibres could be attributed to poor development of the Ca2+ clearance mechanisms. The two components of the transient decay, characterized by the two time constants, could be associated with the operation of the myoplasmic Ca2+-sequestering protein parvalbumin (PV) and of the sarcoplasmic reticulum (SR) Ca2+ ATPase activity respectively. Poisoning of the SERCA pump by cyclopiazonic acid reveals the presence of an initial, fast, poison insensitive recapture component in adult, which is much reduced in young animals. This is consistent with the notion that PV content is very low at birth and increases during postnatal development (Leberer & Pette, 1986).
Interestingly, robust Ca2+ transients appear in muscle fibres early after birth (6-7 days) indicating that at this time the mechanisms underlying ECC are sufficiently developed to produce sizeable Ca2+ release. This is even though the Ca2+ release units formed by ryanodine (RyR) and dihydropyridine receptors (DHPr) at the level of the junctional region between T-tubules (TT) and SR are completed only 2 or 3 weeks after birth (Franzini-Armstrong, 1991).
In spite of robust Ca2+ release, motility is much reduced and slow in young animals (Close, 1964), indicating that the development of the contractile apparatus is the limiting factor. The slowness of movement in young animals may be caused by a poorly developed Ca2+ uptake systems, both PV and SR Ca2+ ATPase, and also by the the fact that the myosin isoform in young animals is of the slow muscle type (Whalen et al. 1981). Thus it is clear that the capacity to develop Ca2+ transients is acquired prior to the complete development of structures involved in contractile activation, in particular myofibrils. This in turn indicates that Ca2+ signals may have a role in myofibrillogenesis (Li et al. 2005).
Acknowledgement
Supported by FONACIT Grant S1-2000000504.
References
Beam KG & Knudson CM. (1988). Effect of postnatal development on Calcium currents and slow charge movement in mammalian skeletal muscle. J Gen Physiol 91, 799-815.
Capote J, Bolaños P, Schuhmeier RP, Melzer W and Caputo C (2005). Calcium transients in developing mouse skeletal muscle fibres. J Physiol 564, 451-464.
Close R (1964). Dynamic properties of fast and slow skeletal muscle of the rat during development. J Physiol 173, 74-95.
Franzini-Armstrong C (1991). Simultaneous maturation of transverse tubules and sarcoplasmic reticulum during muscle differentiation in the mouse. Dev Biol 146, 353-363.
Leberer E & Pette D (1986). Neural regulation of parvalbumin expression in mammalian skeletal muscle. Biochem J 235, 67-73.
Li H, Cook JD, Terry M, Spitzer NC & Ferrari MB (2004). Calcium transients regulate patterned actin assembly during myofibrillogenesis. Dev Dyn 229, 231-42.
Whalen RG, Sell SM, Butler-Browne GS, Schwartz K, Bouveret, P & Pinset-Harstom I (1981). Three myosin heavy-chain isozymes appear sequentially in rat muscle development. Nature 292, 805809.
