
Physiology News Magazine
Can you make a sphincter out of skeletal muscle?
An artificial sphincter is one of the holy grails of bioengineering. Theoretical arguments and experimental studies show that the barriers to progress can be overcome.
Features
Can you make a sphincter out of skeletal muscle?
An artificial sphincter is one of the holy grails of bioengineering. Theoretical arguments and experimental studies show that the barriers to progress can be overcome.
Features
Stanley Salmons, Michael F Russold, Ian Ramnarine, Zoe Ashley, Hazel Sutherland and Jonathan C Jarvis
Department of Human Anatomy and Cell Biology, The University of Liverpool, Liverpool, L69 3GE, UK
https://doi.org/10.36866/pn.79.23

The need
In conditions such as colorectal cancer, Crohn’s disease, ulcerative colitis and inflammatory bowel disease it is frequently necessary to divert the gut, either temporarily or permanently, through a stoma on the abdomen. According to recent estimates, there are approximately 80,000 to 100,000 stoma patients in the UK alone, with 10,000 to 15,000 new stomas created every year. In a high proportion of cases the procedure has a negative impact on quality of life. Patients report leakage, skin rashes, embarrassing noise and odours, interference with sexual activity, and psychological problems arising from the disturbance to body image. Efforts to deal with these problems have met with only isolated success.
What patients need is some way of exerting control over the passage of intestinal contents through the stoma. The biological solution that has been considered for this is an artificial sphincter configured from a body muscle and made to contract and relax by an implanted stimulator. The ideal candidate for this would be the rectus abdominis muscle, which is already used by reconstructive surgeons. The loss of function is well tolerated and, although the donor site is weakened, hernias are rare and can be avoided by applying a reinforcing mesh. This, and the fact that the rectus muscle is well placed for wrapping around the intestine before it emerges through the abdominal wall, makes the surgery entirely feasible. The idea has
been around for a number of years (Konsten et al. 1993), so why isn’t the procedure available?
The wrong kind of muscle?
In the body, the walls of tubular and hollow structures typically contain smooth muscle. This is used to regulate diameter (e.g. blood vessels, branches of the bronchial tree), to propel liquids or solids (e.g. ureter, hepatic duct and intestines) or to expel contents (e.g. urinary bladder, uterus). Smooth muscle is suited to this role for two main reasons:
(a) Its structural organization enables it to contract over a great range, as much as 80% (the urinary bladder, for example, can empty completely from an internal volume of 300 ml). As skeletal muscle is sarcomeric, contraction is limited to a maximum of about 25%. Although this type of muscle develops high forces, range of motion must be achieved through the bony levers of the skeleton.
(b) Smooth muscle can maintain tension for long periods with little expenditure of energy. Skeletal muscle will fatigue under such conditions.
Rectus abdominis is a skeletal muscle, so isn’t any attempt to configure it as a sphincter doomed at the outset?
Range of motion: a question of geometry
Consider a sphincter formed by wrapping skeletal muscle around a segment of intestine with the fibres at right-angles to the long axis of the intestine. When the muscle contracts, the radius shortens in the same proportion as the circumference (circumference = 2πr). Thus a fractional shortening Δ in the fibres reduces the radius to r(1 – Δ). For example, if Δ = 25% and the radius is 7 mm (a realistic intestinal dimension), contraction reduces the radius to 5.25 mm, leaving a patent lumen of 10.5 mm diameter. It appears that a skeletal muscle will never make an effective sphincter. But look again, this time taking account of muscle mechanics.
The muscle wrap has a wall thickness d and an internal radius ri, corresponding to the external diameter of the intestinal wall, so the external radius ro is given by:
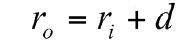
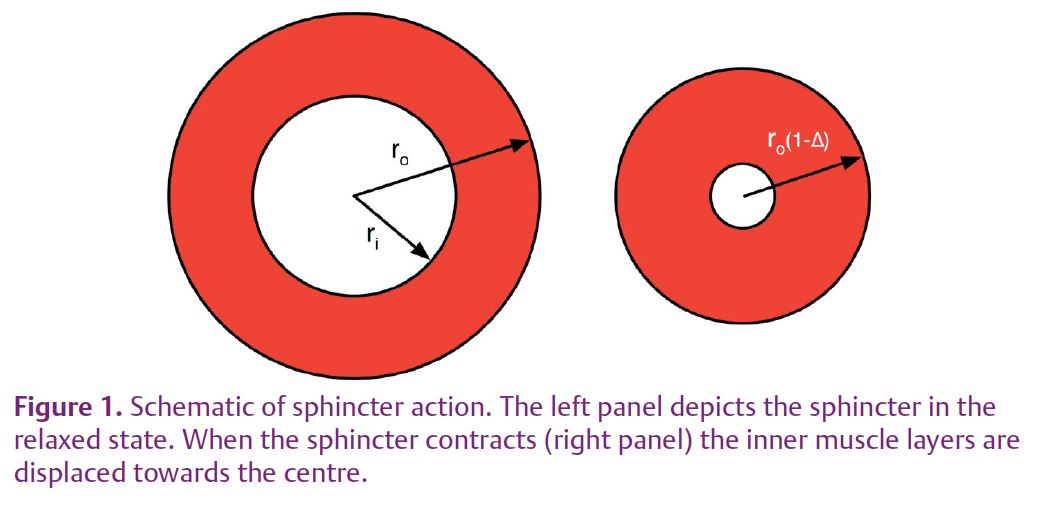
Thick-walled pressure vessel theory tells us that wall stress is greatest at the inside radius and declines through the thickness of the wall. The outermost muscle fibres of the wrap are therefore the least heavily loaded and, since lightly loaded muscle contracts more quickly, these fibres will shorten more than those adjacent to the gut. During contraction, then, the outer layers of the wrap will displace the bulk of the muscle inwards (Fig. 1).
Muscle volume is proportional to cross-sectional area for a uniform cylindrical wrap. If we equate the cross-sectional areas of an open sphincter and an obliterated (closed) sphincter, we get:
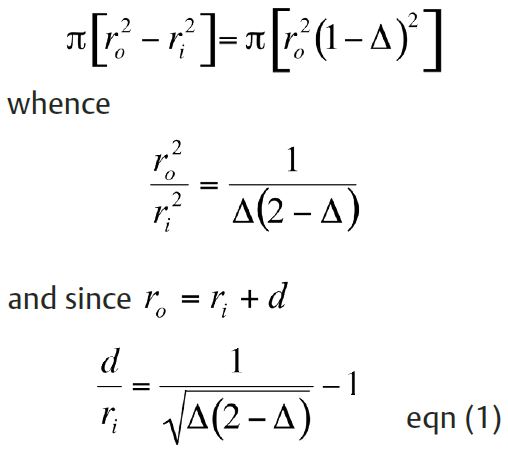
Taking the same example of an intestinal diameter of 7 mm and a fractional shortening of 25%, eqn (1) shows that total closure can, in fact, be achieved if the wall thickess is not less than 3.8 mm. In general, for a given fractional shortening, the greater the diameter of the lumen, the thicker the sphincter needed to close it. The thickness of the rectus abdominis muscle is less than 3.8 mm, so for the sphincter to be effective the muscle must be wrapped twice. Theory therefore predicts that the range of contraction need not be an insuperable problem.
Avoiding fatigue
Closing the intestinal lumen in a single contraction is only one step; to maintain continence a sphincter must be capable of maintaining tension for hours. Certainly a body muscle such as rectus abdominis would fatigue under these conditions. There are, however, two tricks that enable even this problem to be overcome.
Skeletal muscles are capable of adapting to a sustained increase in use. The most familiar example of this is the response to endurance exercise – such as the training undertaken by a marathon runner – as a result of which the muscles become more resistant to fatigue. A more profound transformation can be elicited by chronic electrical stimulation (Salmons & Henriksson, 1981; Salmons, 2009). This procedure, often referred to as ‘conditioning’, activates regulatory phenomena at the gene level which are reflected in muscle physiology, biochemistry and microanatomy at both light and electron microscopic levels (reviewed in Salmons, 2009). In the present context, the relevant features are a slowing of contractile speed and a switch to oxidative metabolic pathways supported by an enhanced blood supply. The latter can be exploited to enhance viability by stimulating the muscle in situ before lifting it as a graft (Tang et al. 1998).
Thus, a conditioned skeletal muscle graft has properties adapted to continuous use. The metabolic changes allow the muscle to obtain energy continuously from oxygen and nutrients in the blood, and the slow contractile speed enables it to develop adequate force at low frequencies of stimulation, which are less demanding of energy.
There is, however, a problem: the constant, high pressure within the sphincter wall will obstruct the muscle’s own blood supply and render it inviable. What can be done about that?
A possible solution would be to interrupt the contraction briefly to allow for reperfusion of the muscle. However, it turns out that even a conditioned muscle relaxes so rapidly that an interruption as brief as 100 ms is enough to lose continence (Russold et al. 2010).
Fortunately the forces required are not so large that the whole muscle needs to be recruited at one time. If different segments of the muscle are activated sequentially, closure can be maintained by one segment while blood is flowing back into the adjacent segment (Zonnevijlle et al. 2000; Russold et al. 2010). The rectus muscle is innervated by several nerves, which are fine and easily damaged. The procedure could be made surgically more robust by using epimysial electrodes but would this provide sufficient separation?
Experimental studies
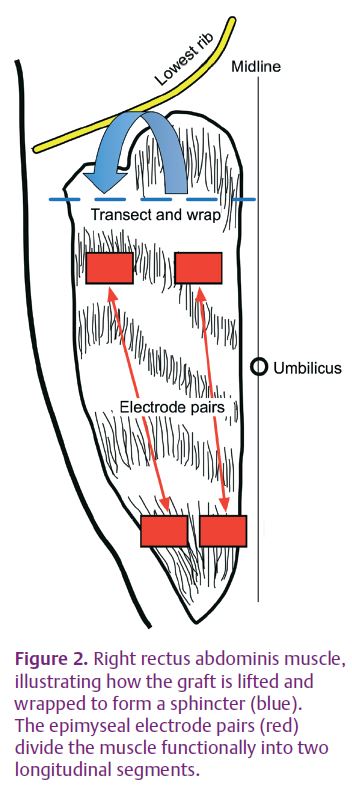
We started by confirming, on an human cadaver, the practicality of constructing a sphincter from the rectus abdominis muscle. We went on to test the ideas outlined above experimentally. Stimulators were implanted in six pigs and used to precondition the rectus abdominis muscle of one side at 2 Hz continuously (Sutherland et al. 2006; Salmons, 2009). The contralateral muscle was used as an unconditioned control. After 4 weeks each muscle was mobilized by dividing it at the upper (rostral) end without disturbing the distributed nerves and the blood supply from the inferior epigastric artery (Fig. 2). It was then configured as a sphincter around a segment of intestine and tested for continence against saline at a pressure of 50–60 mmHg (Fig. 3). Stimulating current was directed alternately to longitudinal segments via epimysial electrodes. A full account of the study has been published (Russold et al. 2010), but the results were essentially as follows.
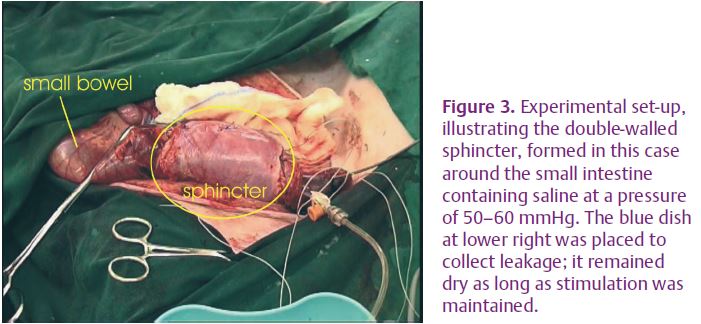
The use of epimysial electrodes provided an acceptable degree of separation of the longitudinal segments. By incorporating a slight overlap between the alternating stimulus pulses, we could maintain pressure continuously within the lumen. There was no leakage of pressurized saline with a double wrap, and occlusion of the lumen could be confirmed by ultrasound imaging. However, when the sphincter was configured with a single wrap it was not continent, confirming the theoretical prediction. Conditioning was essential: unconditioned sphincters could not maintain continence, but sphincters created from the preconditioned muscles were still fully continent after 90 min. At this point we were obliged to terminate the experiments, which had already lasted many hours, but there was every indication that the conditioned, double-wrapped sphincter would have continued to be effective for much longer.
Conclusion
This study removes the remaining objections to configuring a sphincter from the rectus abdominis muscle. If the wall thickness of the sphincter is in the proper relationship to its diameter, complete occlusion is possible. Fatigue can be avoided by a combination of preconditioning and segmental stimulation of intramuscular nerve branches.
Part of the muscle could be left unstimulated to leave a fast-contracting reserve to resist sudden increases in abdominal pressure due to a cough or sneeze. Use of such a device for even a few hours during the day would enable patients to manage their activities more flexibly.
The goal is therefore achievable. In our experience there is no lack of innovative plastic and reconstructive surgeons. All that is needed is a device manufacturer to make an implantable stimulator that is less complicated than a pacemaker.
References
Konsten J, Baeten CG, van Mameren H, Havenith MG & Soeters PB (1993). Feasibility of stoma continence, using electrically stimulated rectus abdominis muscle in pigs. Dis Colon Rectum 36, 247–253.
Russold MF, Ramnarine I, Ashley Z, Sutherland H, Salmons S & Jarvis JC (2010). Practical and effective stomal sphincter creation: evaluation in pigs. Dis Colon Rectum 53, 467–474.
Salmons S (2009). Adaptive change in electrically stimulated muscle: a framework for the design of clinical protocols (Invited review). Muscle Nerve 40, 918–935.
Salmons S & Henriksson J (1981). The adaptive response of skeletal muscle to increased use. Muscle Nerve 4, 94–105.
Sutherland H, Salmons S, Ramnarine IR, Capoccia M, Walsh AA & Jarvis JC (2006). Adaptive conditioning of skeletal muscle in a large animal model (Sus domesticus). J Anat 209, 165–177.
Tang ATM, Jarvis JC, Hooper TL & Salmons S (1998). Observation and basis of improved blood flow to the distal latissimus dorsi muscle: a case for electrical stimulation prior to grafting. Cardiovasc Res 40, 131–137.
Zonnevijlle ED, Somia NN, Abadia GP, Stremel RW, Maldonado CJ, Werker PM, Kon M & Barker JH (2000). Sequential segmental neuromuscular stimulation reduces fatigue and improves perfusion in dynamic graciloplasty. Ann Plast Surg 45, 292–297.
