
Physiology News Magazine
Cardiac channelopathies studied with the dynamic action potential clamp technique
Features
Cardiac channelopathies studied with the dynamic action potential clamp technique
Features
Géza Berecki & Antoni C G van Ginneken
Department of Experimental Cardiology, Academic Medical Center, University of Amsterdam, The Netherlands
https://doi.org/10.36866/pn.63.28

The congenital long-QT syndrome (LQTS) is an inherited disorder that prolongs repolarisation of the ventricular myocyte. It often leads to sudden death from cardiac arrhythmias arising from oscillations during the action potential (AP) plateau, specifically torsades de pointes and ventricular fibrillation. It is a characteristic feature of the cardiac cell membrane that even small changes in individual ionic currents can shift the delicate balance between inward and outward current flow during the plateau and repolarisation and thereby prolong or shorten the AP.
Since the discovery of genetic linkage between cardiac ion channels and the LQTS in the mid 1990s, remarkable results were obtained in understanding various types of this syndrome. At present, most effort has been devoted to documenting the effects of identified mutations on the densities and kinetics of various cardiac ion channels upon heterologous expression. Subsequently, the consequences of channelopathies for cardiac function are inferred by testing the functional effects of the experimentally observed changes with use of mathematical models of cardiac cells (Wehrens et al. 2002). However, despite advances in mathematical modeling, the mechanism by which a given genetic defect leads to the clinically observed electrical disease often remains obscure: genetic heterogeneity in LQTS represents a major challenge for modelers as various mutations in the same gene may produce different, often inconsistent functional effects.
The recently introduced ‘dynamic action potential clamp’ (dAPC) technique (Berecki et al. 2005; 2006) represents an alternative approach to investigate LQTS without making assumptions with regard to (altered) kinetic properties of the studied channel(s). As an extension of the dynamic clamp methodology (briefly reviewed by Wilders, 2005), allows the study of various types of LQTSs (e.g. LQT2 and LQT3, which are linked to mutations in the human ether-a-go-gorelated gene, HERG, and sodium channel gene, SCN5A, respectively).
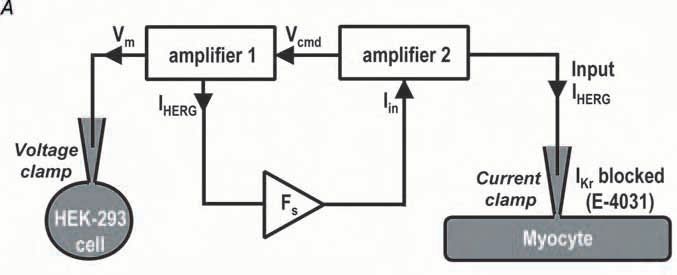
The concept of dAPC is that a selected native ionic current of a ventricular myocyte is effectively replaced with wild-type or mutant current recorded from a transiently transfected human embryonic kidney (HEK)-293 cell. During the dAPC experiment, the ventricular cell and the HEK-293 cell are electrically coupled by means of an electrical circuit. The ventricular cell is in ‘current clamp’ mode on one patchclamp setup, whereas the HEK293 cell is in ‘voltage clamp’ mode on another setup. The command potential for the HEK293 cell is the Vm of the ventricular cell (‘action potential clamp’), and the current input applied to the ventricular cell is the current recorded from the transfected HEK293 cell, a connection resulting in dAPC condition.
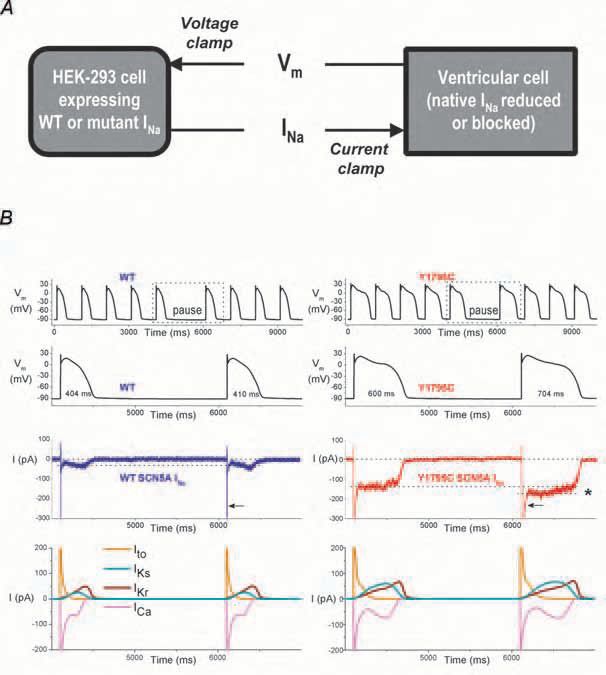
Both a computed model of the human ventricular cell (Priebe & Beuckelmann, 1998) (Fig. 1) or a freshly isolated (real) myocyte (Fig. 2) can effectively be used in dAPC experiments, defining the ‘model cell’ and ‘real cell’ modes, respectively. The first offers an outstanding reproducibility of the results, because the implemented (input) wild-type (WT) or mutant current is the only variable during experimentation. Moreover, it allows ‘generation’ of subendocardial, midmyocardial (M), and subepicardial ventricular cells by adjusting selected membrane ionic currents in the ventricular model cell. Equally, the technically more difficult real cell mode reveals AP waveforms and ion channel kinetics that can be considered close-to-physiological.

The dAPC approach allows rapid and unambiguous determination of the effect(s) of ion channel mutation on the ventricular AP morphology. During experiments, the (altered) shape of the AP directly reflects the effect of a mutation; the frequency dependence of the AP durations, the consequence of a pause on AP morphology as well the arrhythmogenic nature of LQTassociated mutations can be determined, and special kinetic features of cardiac channels can be revealed. With adequate scaling adjustments and procedures to reduce unwanted and contaminating background currents, this novel technique allows several cardiac ion channels to be studied.
The dAPC technique represents a promising new tool to study various cardiac ion channels and may also prove useful in related fields of research, e.g. in neurophysiology.
Acknowledgement
This study was supported by Netherlands Heart Foundation Grant 2001B155.
References
Berecki G, Zegers JG, Verkerk AO, Bhuiyan ZA, de Jonge B, Veldkamp MW, Wilders R & van Ginneken AC (2005). HERG channel (dys)function revealed by dynamic action potential clamp technique. Biophys J 88, 566-578.
Berecki G, Zegers JG, Bhuiyan ZA, Verkerk AO, Wilders R, & van Ginneken AC (2006). Long-QT syndrome-related sodium channel mutations probed by the dynamic action potential clamp technique. J Physiol 570, 237-250.
Priebe L & Beuckelmann DJ (1998). Simulation study of cellular electric properties in heart failure. Circ Res 82, 1206-1223.
Wehrens XH, Vos MA, Doevendans PA & Wellens HJ (2002). Novel insights in the congenital long QT syndrome. Ann Intern Med 137, 981-992.
Wilders R (2005). ‘Dynamic clamp’ in cardiac electrophysiology. J Physiol 566, 641
