
Physiology News Magazine
Central CO2 chemoreception: how can it be done without the perfect receptors?
Central CO2 chemoreception is a vital sensory function believed to be carried out by specific CO2 chemoreceptors. Recent studies suggest that brainstem neuronal networks may be able to achieve high CO2 sensitivity and a wide sensing spectrum by common pH-sensitive ion channels and synaptic interactions
Features
Central CO2 chemoreception: how can it be done without the perfect receptors?
Central CO2 chemoreception is a vital sensory function believed to be carried out by specific CO2 chemoreceptors. Recent studies suggest that brainstem neuronal networks may be able to achieve high CO2 sensitivity and a wide sensing spectrum by common pH-sensitive ion channels and synaptic interactions
Features
Chun Jiang, Junda Su,& Asheebo Rojas
Department of Biology, Georgia State University, Atlanta, Georgia, USA
https://doi.org/10.36866/pn.68.23
Only a handful of sensory functions remain without cellular identity. Central CO2 chemoreception is one of them. Since it was first described almost a half century ago, tremendous efforts have been devoted to identify the central CO2 chemoreceptors (CCRs). We now know their likely locations in certain brainstem nuclei in which several types of CO2-chemosensitive neurons have been observed. We even know some potential cellular and molecular mechanisms for CO2 sensing. However, the identity of the CCRs remains uncertain. As a result, several phenomena observed in central CO2 chemoreception are rather controversial.
The ideal CCR cells would be highly sensitive to CO2, project to respiratory-modulated neurons, and dictate systemic CO2 responses. However, experimental evidence supporting the existence of such typical sensory cells is lacking. In contrast, neurons with various CO2 chemosensitivity are found in many parts of the medulla and pons, although their functional significance is still not fully understood
(Richerson, 2004).
The search for molecular CO2/pH sensors has led to a number of candidate molecules in the past decade (Jiang et al. 2005). Studies involving transgenic mice, however, indicate that CO2 chemoreception appears normal in animals lacking one or other of these putative sensing molecules, such as 5-HT2a receptor, TASK-1 channel and P2x receptor is disrupted (Linden et al. 2006; Popa et al. 2005; Rong et al. 2003). This raises the question as to how the results from previous physiological studies on these sensing molecules can be explained.
Perhaps the most paradoxical phenomenon in central CO2 chemoreception is how the high CO2 sensitivity and a wide sensing spectrum are achieved. The respiratory neuronal networks are highly sensitive to CO2 over a wide sensing range (pCO2 20–90 mm Hg), allowing a 20–30% change in systemic ventilation with a minute increase (by 1–2 mm Hg) in pCO2 (Feldman et al. 2003). The high sensitivity and wide sensing spectrum cannot be produced by a single pH sensing molecule.
As shown in Fig. 1, the pH sensitivity of a molecule is determined by the steepness of its pH titration curve. The steeper the curve (corresponding to a high ‘h’ value in the Hill equation), the narrower the pH-sensing range. Thus, a highly pH-sensitive molecule tends to work in a rather narrow pH range. On the other hand, the sensitivity becomes low if it senses pH in a broad range. Therefore, the sensor molecule that is supreme in both sensitivity and spectrum does not seem to exist.
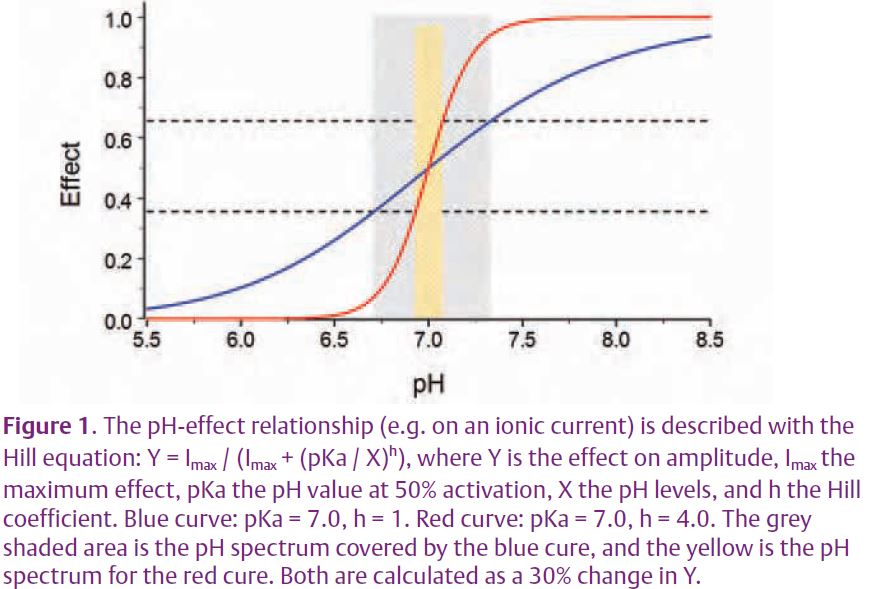
How does the respiratory neuronal network resolve the high sensitivity versus wide spectrum paradox? To address this, it is necessary to have a preparation that allows observation, as well as manipulation, of the cell-intrinsic membrane properties and neuronal interactions in their networks. For the latter simultaneous recordings from multiple neurons are essential. Thus, we have made primary neuronal cultures in a multi-electrode array (MEA) dish. Still in its infancy, the preparation has several weaknesses such as the loss of anatomical identity and the reduction in network components. Despite these problems, it is a useful preparation for the studies of both intrinsic membrane properties and synaptic interactions as well as simultaneous and repetitive recordings from multiple neurons.
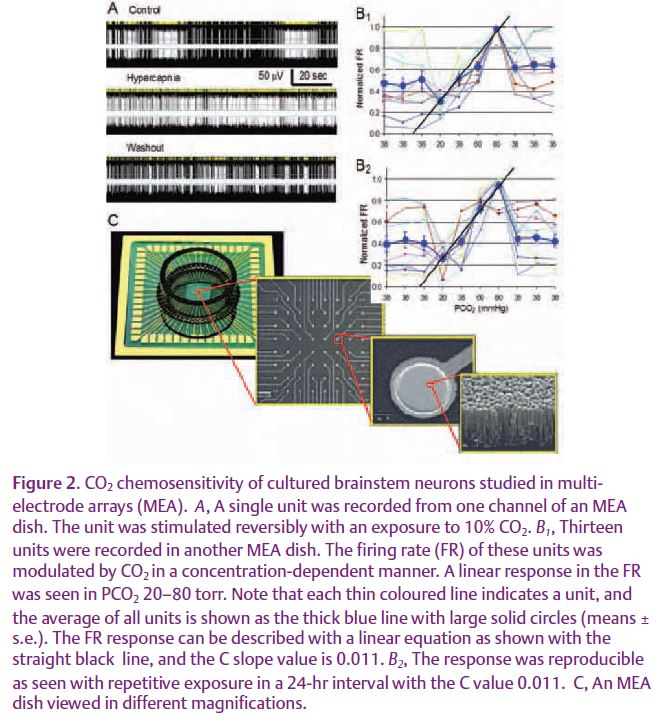
Using the MEA technique, we studied the paradox of high sensitivity versus broad spectrum. We have found that cultured brainstem neurons retain their high CO2 chemosensitivity in a broad sensing range in the MEA dish (Fig. 2). Blockade of inward rectifier K+ (Kir) channels disrupts neuronal sensitivity to moderate hypercapnia and hypocapnia, whereas inhibition of TASK channels abrogates the neuronal response to more severe hypercapnia. These are consistent with the pH sensitivities of the heteromeric Kir4.1-Kir5.1 and the TASK-1 channels, both of which are expressed in the brainstem, suggesting that the broad PCO2 spectrum can be covered by multiple sensors (Su et al. 2007). Since these molecules work in parallel for the detection of PCO2 levels in the same cells, we termed this sensing the ‘parallel process’ (Fig. 3).
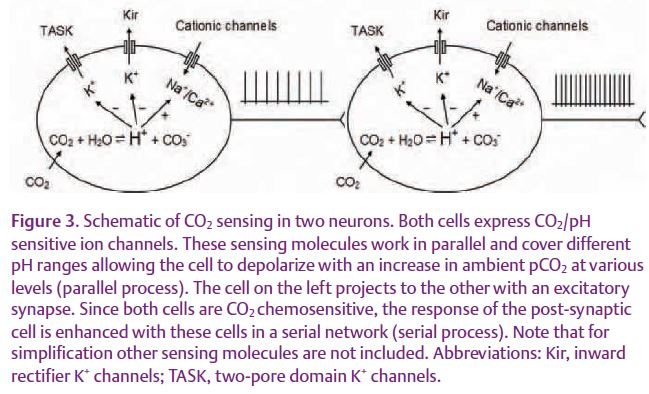
Although the parallel arrangement of multiple sensors helps neurons to preserve the CO2 sensitivity of each sensor, these K+ channels do not appear to be sensitive enough to transduce a change of 1–2 mm Hg PCO2 into a 20–30% change in cellular activity. We have calculated the possible effect of the pH-sensitive Kir channels on membrane potentials, and found that at best they can produce a depolarization of ~1.5mV / 1 mm Hg PCO2. Clearly, the high CO2 sensitivity may not be produced by the primary sensory neurons alone. Therefore, we included neuronal network properties in our studies. We have found that CO2 chemosensitivity is greatly reduced when neurons are isolated from their networks, suggesting that the CO2 chemo-sensitivity relies on not only the cell-intrinsic sensing mechanism, but also on neuronal interactions through synaptic transmission. Indeed, our results show that several major excitatory neurotransmitters critical for respiratory control are involved in chemosensing synaptic transmission (Su et al. 2007). With intact network connections, some neurons increase their firing rate by 20–30% with a rise in1 mm Hg PCO2, suggesting that an amplification mechanism by pre- and post-synaptic neurons exists, which we termed the ‘serial process’ (Fig. 3).
With the demonstration of the parallel and serial processes of CO2 chemosensitivity, we may find explanations for other controversial phenomena:
- why are the optimal CCRs hard to find? The systemic CO2 response can be fulfilled by neurons in several CO2-chemosensitive nuclei and their synaptic connections with respiratory neurons through signal amplification, but may not be accomplished by individual cells without the signal amplification;
- why are there so many CO2-chemosensitive neurons? The signal amplification may involve a large number of interneurons, motor neurons and peripheral sensory neurons as well if they are CO2-chemosensitive;
- why does knockout of a likely CO2 sensing molecule impose very few adverse consequences to the system? The overlap in the spectrum of the sensing molecules may allow them to compensate for the functional defect of each other, a likely evolutional strategy for the reservation of certain vital functions such as control of respiration.
Of course, these observations need to be verified in other preparations, as they were made in an in vitro preparation of reduced neuronal networks. It is the reduced network preparation however that allows us to make a leap from individual cells to neuronal networks. We believe that with the emphasis on both individual neurons in several brainstem CO2 chemosensitive nuclei and the neuronal networks, better preparations can be developed, which will surely lead to an improved understanding of central CO2 chemoreception.
References
Feldman JL, Mitchell GS & Nattie EE (2003). Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci 26, 239–266.
Jiang C, Rojas A, Wang R & Wang X (2005). CO2 central chemosensitivity: why are there so many sensing molecules? Respir Physiol Neurobiol 145, 115–126.
Linden AM, Aller MI, Leppa E, Vekovischeva O, Aitta-Aho T, Veale EL, Mathie A, Rosenberg P, Wisden W & Korpi ER (2006). The in vivo contributions of TASK-1-containing channels to the actions of inhalation anesthetics, the alpha(2) adrenergic sedative dexmede-tomidine, and cannabinoid agonists. J Pharmacol Exp Ther 317, 615–626.
Popa D, Lena C, Fabre V, Prenat C, Gingrich J, Escourrou P, Hamon M & Adrien J (2005). Contribution of 5-HT2 receptor subtypes to sleep-wakefulness and respiratory control, and functional adaptations in knock-out mice lacking 5-HT2A receptors. J Neurosci 25, 11231–11238.
Richerson GB (2004). Serotonergic neurons as carbon dioxide sensors that maintain pH homeostasis. Nat Rev Neurosci 5, 449–461.
Rong W, Gourine AV, Cockayne DA, Xiang Z, Ford AP, Spyer KM & Burnstock G (2003). Pivotal role of nucleotide P2X2 receptor subunit of the ATP-gated ion channel mediating ventilatory responses to hypoxia. J Neurosci 23, 11315–11321.
Su J, Yang L, Zhang X, Rojas A, Shi Y & Jiang C (2007). High CO2 chemosensitivity versus wide sensing spectrum: a paradoxical problem and its solutions in cultured brainstem neurons. J Physiol 578, 831–841.
