
Physiology News Magazine
Channel gating in the LGIC receptor superfamily – insights into the signal
Recent papers on ligand-gated ion channels reveal more molecular detail on the conformational changes that follow ligand binding and lead to pore opening
Features
Channel gating in the LGIC receptor superfamily – insights into the signal
Recent papers on ligand-gated ion channels reveal more molecular detail on the conformational changes that follow ligand binding and lead to pore opening
Features
Nathan L Absalom (1), Trevor M Lewis (2), Peter R Schofield (1)
1: Neurobiology Program, Garvan Institute of Medical Research, Darlinghurst, NSW, Australia.
2: School of Medical Sciences, The University of New South Wales, Sydney, NSW, Australia.
https://doi.org/10.36866/pn.57.27

Understanding receptor function has proved to be a demanding task. Once ligand-gated ion channel (LGIC) receptors were identified as the engines that converted chemical signals into electrical currents at the synapse, the structure of these molecules and how this related to their function became a major topic of fundamental research. Recent advances have led to an unprecedented level of detailed knowledge of the structure of the nicotinic acetylcholine receptor (nAChR). By homology, this structural information has been applied to other members of the cys-loop family of receptors – the glycine, γ-aminobutyric acid type A (GABAA) and serotonin type III (5-HT3) receptors. These receptors are all pentameric complexes with each subunit sharing a common topology of a large extracellular N-terminal domain and four transmembrane domains (M1-M4), of which the M2 lines the channel pore. The merging of molecular structure information and traditional mutational and functional analysis has seen the field leap ahead in our understanding of these receptors.
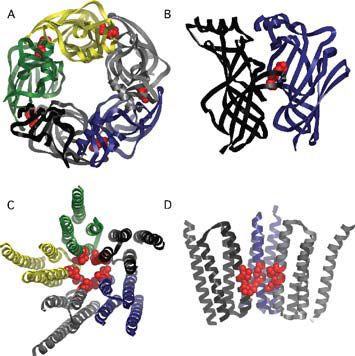
The recent advances in the field have been driven by two important publications. The first was the structure of the acetylcholine binding protein (AChBP) derived from X-ray crystallography (Brejc et al. 2001). The AChBP scavenges acetylcholine molecules at cholinergic synapses in the snail Lymnaea stagnalis and has a high degree of similarity in amino acid sequence to the extracellular domain of the nAChR (Fig. 1A,B). This gave researchers a three-dimensional portrait of the molecular structure of the ligand-binding pocket. Homology structures were built for the extracellular domains of the acetylcholine receptor and the other members of the cys-loop family of receptors to help investigate other questions of receptor function. Of particular interest was the location of the conserved cys-loop and loop 2 of the extracellular domain, which appeared to be in position to interact with the transmembrane domains of the receptor complex.
Using traditional site-directed mutagenesis and electrophysiological techniques, electrostatic interactions were found between a lysine residue in the M2-M3 linker region and acidic residues in the cys-loop and loop 2 that were critical for coupling agonist binding to channel gating in the GABAA receptor (Kash et al. 2003). The homologous structures in the glycine receptor were also found to be critical in this signal transduction process, although the electrostatic interactions were not the same (Absalom et al. 2003). This is thought to be a consequence of different protein sequences in these regions.
The second key publication describes the refined electron micrograph derived structure of the Torpedo AChR, which provides a detailed three-dimensional structure of the transmembrane domains and, in particular, the channel pore (Miyazawa et al. 2003) (Fig. 1C,D). The pore structure indicates that there is a ‘hydrophobic girdle’ formed by conserved residues located in the middle of the M2 domain that constitutes the channel ‘gate’, blocking ion flow in the closed state. Rotation of one or two of the M2 domains would disrupt the hydrophobic interactions with adjacent helices and collapse the hydrophobic girdle, allowing ions to flow through the channel.
From earlier electron density structures of the AChR, it was known that binding of agonist causes rotations in the extracellular domain of the receptor and of the M2 domains (Unwin et al. 2002), but how these events were coupled was not clear. That these conformational events were coupled sequentially to open the channel pore had been demonstrated by Grosman and colleagues (2000). They mapped a ‘conformational wave’ that propagated from the ligand binding site to the channel pore via the M2-M3 linker, as determined by linear free energy relationships. With the refined structure of the nAChR, a molecular framework is provided for these conformational changes which predicts interactions between the conserved cys-loop and the M2-M3 linker (Fig. 2). However, unlike the electrostatic interactions in the GABAA receptor, these interactions are predicted to be hydrophobic in nature in the AChR (Miyazawa et al. 2003).
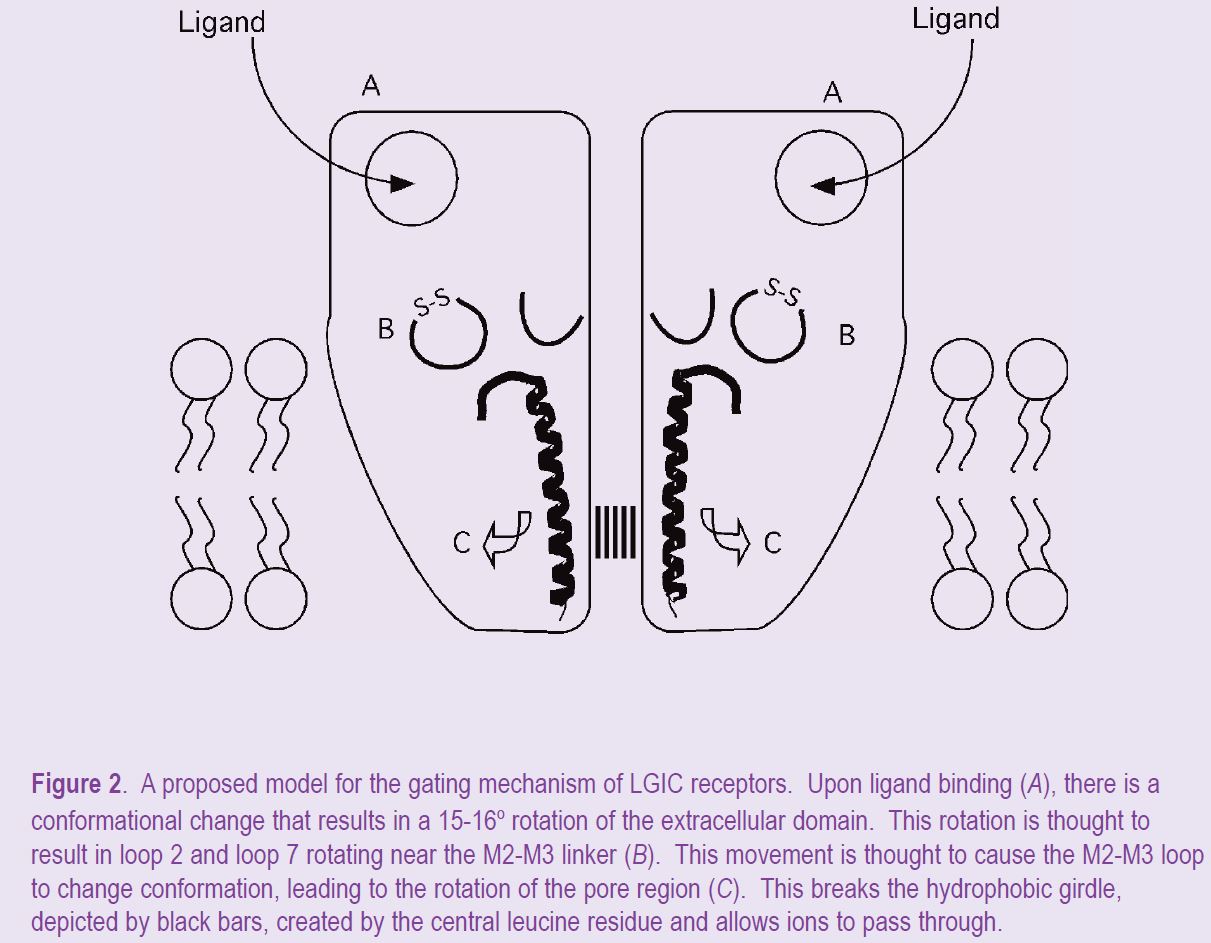
These key publications on the structure of the AChBP and the transmembrane domain of the AChR have provided alluring details of these domains in cys-loop family of LGICs and of the possible interactions at the interface between these domains. Combined with previous information obtained by site-directed mutatgenesis studies, this has resulted in fledgling mechanism that for the first time makes specific structural links between the ligand binding domain and the opening of the channel pore (Fig. 2). There is still a lot of detail to fill in with this mechanism, but it has already identified the important elements of this process.
References
Absalom NL, Lewis TM, Kaplan W, Pierce KD & Schofield PR (2003). Role of charged residues in coupling ligand binding and channel activation in the extracellular domain of the glycine receptor. J Biol Chem 278, 50151-50157.
Brejc K, van Dijk WJ, Klassen RV, Schuurmans M, van Der Oost J, Smit AB & Sixma TK (2001). Crystal structure of an ACh-binding protein that reveals the ligand-binding domain of the nicotinic receptors. Nature 411, 269-276.
Grosman C, Zhou M & Auerbach A (2000). Mapping the conformational wave of acetylcholine receptor channel gating. Nature 403, 773-776.
Kash TL, Jenkins A, Kelley JC, Trudell JR and Harrison NL (2003). Coupling of agonist binding to channel gating in the GABAA receptor. Nature 421, 272-275.
Miyazawa A, Fujiyoshi Y & Unwin N (2003). Structure and gating mechanism of the acetylcholine receptor pore. Nature 423, 949-955.
Unwin N, Miyazawa A, Li J & Fujiyoshi Y (2002). Activation of the nicotinic acetylcholine receptor involves a switch in conformation of the alpha subunits. J Mol Biol 319, 1165-1176.
