
Physiology News Magazine
Chronic micro-inflammation: is it the enemy in the battle for maintaining muscle mass during ageing?
Ageing is characterized by a gradual loss of muscle proteins (sarcopenia), which is ultimately responsible for decreased mobility and autonomy of the elderly. A decreased efficiency of nutrition on skeletal muscle anabolism has been blamed on this deterioration. Containing micro-inflammation that develops during normal ageing may be a good tool to maintain muscle mass and autonomy.
Features
Chronic micro-inflammation: is it the enemy in the battle for maintaining muscle mass during ageing?
Ageing is characterized by a gradual loss of muscle proteins (sarcopenia), which is ultimately responsible for decreased mobility and autonomy of the elderly. A decreased efficiency of nutrition on skeletal muscle anabolism has been blamed on this deterioration. Containing micro-inflammation that develops during normal ageing may be a good tool to maintain muscle mass and autonomy.
Features
Dominique Dardevet and Laurent Mosoni
Unité de Nutrition Humaine, INRA, 1019 Nutrition Humaine, F-63122 Saint Genès Champanelle, France
https://doi.org/10.36866/pn.79.26


Normal ageing is characterised by a decline in skeletal muscle mass and strength associated with increased muscle fatigability (Fig. 1). This phenomenon, named sarcopenia, reduces physical activity and generates a general weakness in elderly men and women. The weakness of quadriceps muscle predisposes to impaired locomotion, frequent falls and increases the risk of hip fractures in the elderly.

In addition, there is an increased susceptibility to illness since skeletal muscles are the major reservoir of body proteins, and consequently of amino acids, which could be used for energy production or the synthesis of acute-phase proteins by the liver. Due to the reduced muscle mass, the ability of aged individuals to fight and recover from stress is impaired. All these factors taken together, sarcopenia reduces the quality of life for the rapidly expanding older population in Western countries. Elucidating the mechanisms that result in muscle wasting in ageing is therefore of obvious importance. It is estimated that $20–30 billion of health costs are spent in the USA on problems directly related to sarcopenia (Schneider & Guralnik, 1990).
Alterations in mechanical and biochemical properties of skeletal muscle are very similar in elderly men and women, and elderly rodents. Muscle proteins undergo a continuous process of degradation and synthesis. Thus, protein storage in skeletal muscle results directly from the overall balance between the rates of protein synthesis and breakdown. Sarcopenia observed during ageing is then the consequence of a decreased protein synthesis, increased proteolysis or a combination of the two phenomena.
Ageing and postprandial muscle protein metabolism
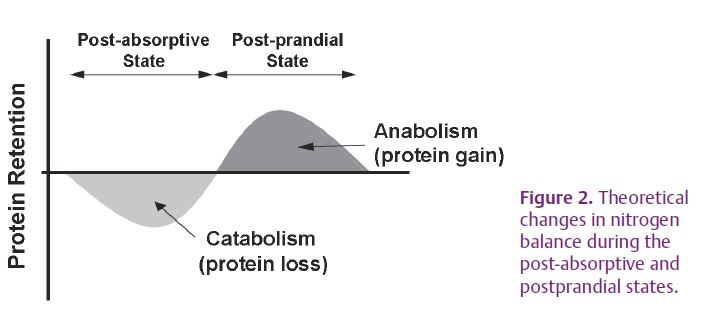
Throughout the day, these activities of protein synthesis and breakdown are not stable and vary according to the nutritional state: whole body proteins are stored after food intake and lost after prolonged period without food (e.g. at night). On a short-term basis in adult, muscle protein mass remains stable and fasting muscle protein loss is compensated by the same protein gain during the feeding period (Fig. 2). These changes are mediated by feeding-induced increases in plasma concentrations of both nutrients and hormones. Many studies suggest that amino acids and insulin play major roles in promoting postprandial protein anabolism. In rats, Mosoni et al. (1995) and Dardevet et al. (2002) found that protein synthesis was stimulated by feeding only in adult but not in elderly rats. This loss of protein synthesis response to the anabolic effect of food intake could be involved in age-related muscle protein loss, since every day fasting-induced protein loss will not be completely recovered during the postprandial period in the oldest subjects. Several studies have indicated that branched-chain amino acids (BCAA: leucine, valine and isoleucine) regulate skeletal muscle protein synthesis and proteolysis. A decrease in muscle protein synthesis and proteolysis sensitivity to leucine during ageing may explain the defect in postprandial anabolism (Combaret et al. 2005; Katsanos et al. 2006; Rieu et al. 2006). The decreased sensitivity of postprandial muscle protein metabolism to leucine in the elderly suggests that the signalling pathway that carries the leucine signal to the protein translation machinery is less responsive to the amino acid than in adults (Dardevet et al. 2000). Increasing dietary intake of leucine has been shown to present beneficial effects on skeletal muscle postprandial anabolism in the elderly (reviewed in Balage et al. 2010).
Sarcopenia and low-grade inflammation
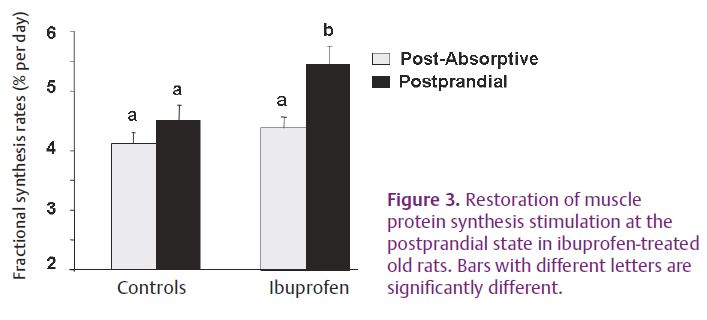
The origin of this resistance to the stimulatory effect of food intake (i.e. dietary amino acid leucine) is unknown and still under question. Ageing may be also characterized by the development of a low-grade inflammatory state. Levels of inflammatory markers, such as interleukin-6 (IL6) and C reactive protein (CRP), increase slightly with ageing, and these higher levels are correlated with disability and mortality in humans. Even if the increase is moderate, higher levels of cytokines and CRP increase the risk of muscle strength loss (Schaap et al. 2006) and are correlated with lower muscle mass in healthy older persons. Cytokines and particularly tumour necrosis factor-α impair skeletal muscle protein synthesis by decreasing the stimulation of the mTOR signalling pathways. Interestingly, this pathway has been shown to be responsible for muscle protein synthesis stimulation by food intake and amino acids. Therefore, a modulation of low-grade inflammation during ageing may be beneficial to improve the response of muscle protein metabolism to food intake. To test this hypothesis, a non-steroidal anti-inflammatory drug (NSAID) which inhibits cyclooxygenase activities, including COX2, was used (Ibuprofen). It significantly increased fed state muscle protein synthesis by 25% and significantly decreased proteolysis in a rat model (Fig. 3). The restoration of muscle protein anabolism during the postprandial state by controlling the development of low-grade inflammation significantly decreased sarcopenia (Rieu et al. 2009).
Interestingly, an antioxidant supplementation has been shown to reduce the inflammation state and/or oxidative stress in the elderly and has also been shown to improve the ability of leucine to stimulate protein synthesis in muscles of old rats independently of an increase in leucine availability (Marzani et al. 2008).
Conclusion
Our recent observations have identified low-grade inflammation as an important target for pharmacological, nutritional and lifestyle interventions that aim to limit sarcopenia and muscle weakness in the rapidly growing elderly population.
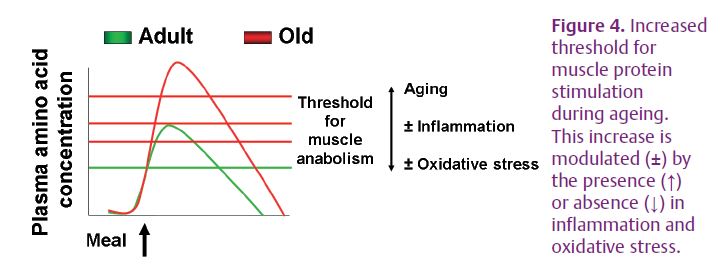
In order to slow down the loss of muscle proteins during ageing, it is necessary to take into account the higher threshold for the stimulation of muscle protein anabolism. It is possible that eating more protein/amino acids/leucine will enable older individuals to reach the plasma amino acid concentration corresponding to this higher threshold. It also seems possible that pharmacological (ibuprofen), nutritional (fruits and vegetables) or lifestyle (exercise) interventions that reduce low-grade inflammation, could lower the threshold for protein metabolism in the elderly (Fig. 4).
References
Balage M & Dardevet D (2010). Long-term effects of leucine supplementation on body composition. Curr Opin Clin Nutr Metab Care Jan 25. [Epub ahead of print]
Combaret L, Dardevet D, Rieu I, Pouch MN, Bechet D, Taillandier D et al. (2005). A leucine-supplemented diet restores the defective postprandial inhibition of proteasome dependent proteolysis in aged rat skeletal muscle. J Physiol 569, 489–499.
Dardevet D, Sornet C, Balage M & Grizard J (2000). Stimulation of in vitro rat muscle protein synthesis by leucine decreases with age. J Nutr 130, 2630–2635.
Dardevet D, Sornet C, Bayle G, Prugnaud J, Pouyet C & Grizard J (2002). Postprandial stimulation of muscle protein synthesis in old rats can be restored by a leucine-supplemented meal. J Nutr 132, 95–100.
Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A & Wolfe RR (2006). A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am J Physiol Endocrinol Metab 291, E381–E387.
Marzani B, Balage M, Vénien A, Astruc T, Papet I, Dardevet D & Mosoni L (2008). Antioxidant supplementation restores defective leucine stimulation of protein synthesis in skeletal muscle from old rats. J Nutr 138, 2205–2211.
Mosoni L, Valluy MC, Serrurier B, Prugnaud J, Obled C, Guezennec CY & Patureau Mirand P (1995). Altered response of protein synthesis to nutritional state and endurance training in old rats. Am J Physiol Endocrinol Metab 268, E328–E335.
Rieu I, Balage M, Sornet C, Giraudet C, Pujos E, Grizard J, Mosoni, L & Dardevet D (2006). Leucine supplementation improves muscle protein synthesis in elderly men independently of hyperaminoacidaemia. J Physiol 575, 305–315.
Rieu I, Magne H, Savary-Auzeloux I, Averous J, Bos C, Peyron MA, Combaret L & Dardevet D (2009). Reduction of low grade inflammation restores blunting of postprandial muscle anabolism and limits sarcopenia in old rats. J Physiol 587, 5483–5492. http://jp.physoc.org/content/587/22/5483.long
Schaap LA, Pluijm SMF, Deeg DJH & Visser M (2006). Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am J Med 119, e9–e17.
Schneider EL & Guralnik JM (1990). The aging of America. Impact on health care costs. JAMA 263, 2335–2340.
