Physiology News Magazine
Contribution of myoglobin facilitated O2 diffusion in respiring tissue
Features
Contribution of myoglobin facilitated O2 diffusion in respiring tissue
Features
Ping Chang Lin, Ulrike Kreutzer, & Thomas Jue
Biochemistry and Molecular Medicine, University of California Davis, Davis, CA, USA
https://doi.org/10.36866/pn.69.39

Sometimes scientists stumble innocently into a research question and then discover that the results do not match the accepted views. Hand wringing usually rues the naivety, while peers rush to explain away the new observations with methodology doubts. But if the observations hold, a new idea can rise.
For many, the function of myoglobin (Mb) poses no question. All biochemistry textbooks assert that Mb stores or transports oxygen. The view seems reasonable, given its structural features, its O2 binding, and its homology with hemoglobin (Hb). Indeed, Kendrew and Perutz established a field of protein crystallography by describing the structure of these heme proteins. Monod, Wyman, and Changeux explained O2 binding to Hb with a two state model paradigm. Given the tomes of experimental data, Mb or Hb structure or function should stand on a solid conceptual framework.
But Wyman actually puzzled over Mb function in the cell. Its concentration in terrestrial mammals seemed too low to serve as an adequate oxygen store. He proved mathematically a theory proposed by Wittenberg. Namely, Mb can compete effectively with free O2 in delivering oxygen to the mitochondria. In essence the high O2 carrying capacity of Mb overcomes the low solubility of free O2. Two assumptions underpin the hypothesis: cellular O2 in blood perfused tissue only partially saturates Mb, and cellular Mb must have sufficient mobility to compete with free O2 diffusion. At high O2 levels, as characterized by a fully saturated Mb, the diffusion of free O2 will transport more efficiently than Mb. If Mb diffuses too slowly, it cannot compete effectively with free O2 diffusion.
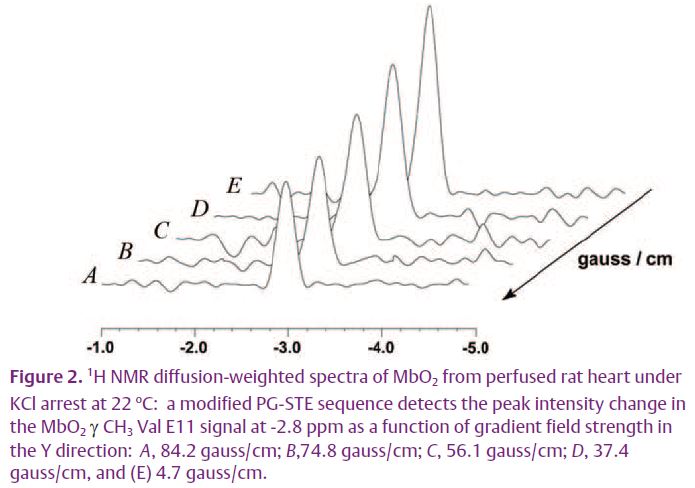
The Mb facilitated O2 diffusion hypothesis, however, has languished for definitive experimental verification for over 40 years. Nevertheless, these ideas about Mb function form the cornerstone of current respiratory control models. Because 1H NMR has now detected the distinct γ CH3 Val E11 and proximal histidyl NδH signals of Mb in myocardium and skeletal muscle, an opportunity has emerged to determine cellular PO2 and to apply pulsed-field gradient techniques to map endogenous Mb translational diffusion (Kreutzer et al. 1992; Stejskal & Tanner, 1965).
In the basal state, 1H NMR has not detected any deoxy Mb proximal histidyl NδH signal in perfused or in situ myocardium. In contrast, the Mb facilitated diffusion hypothesis envisions a partially saturated Mb in blood perfused tissue. Even as work doubles, the in situ myocardium shows no signs of Mb desaturation (Kreutzer et al. 2001; Zhang et al. 1999).
What about the other assumption in the Mb facilitated diffusion hypothesis? Solution experiments confirm that the NMR determined diffusion coefficients of 11.6 x 10-7 cm2/s (Mb) and 7.53 x 10-7 cm2/s (Hb) agree closely with literature reported values. In respiring rat myocardium, Mb diffusion drops by 37% to 4.24 x 10-7 cm2/s. The cellular DMb matches the value predicted by the NMR rotational diffusion analysis (Wang et al. 1997).
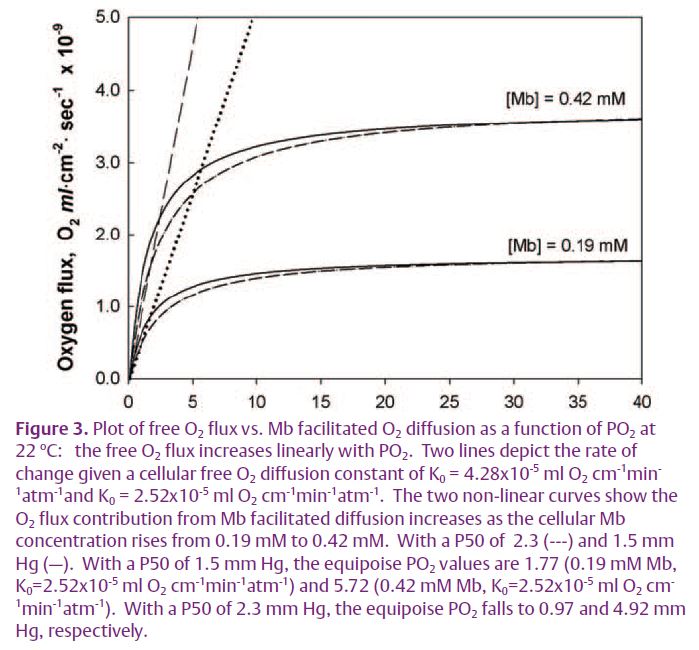
Given the cellular DMb, the cellular Mb concentration, the Mb P50 (PO2 that half saturates Mb), and K0 (the diffusion of free O2 in the cell), the data analysis can assess the relative contribution of the O2 flux from Mb (FO2 Mb) vs. from free O2 (FO2 O2), as defined by an equipoise diffusion PO2, the PO2 in which Mb and free O2 contribute equally to the cellular O2 flux. For the rat heart, the equipoise PO2 stands around 1.8 mm Hg. Below a PO2 of 1.8 mm Hg, the Mb dependent contribution to the O2 flux dominates. Because the NMR observation indicates that the basal state in situ myocardium operates with a PO2 above 10 mm Hg, Mb cannot play a significant role in facilitating O2 transport under normal physiological conditions in the myocardium.
The concentration of Mb alters significantly the equipoise PO2. In terrestrial mammalian muscle, the Mb concentration ranges from 0.19 mM in heart muscle to 0.42 mM in soleus muscle. Soleus muscle has then a higher equipoise PO2 than myo-cardium. In fact, marine mammalian muscle with Mb concentration up to 25-70 g/kg tissue (approximately 1.4-4 mM) would have an equipoise PO2 of 12-38 mm Hg. With marine mammals, Mb dependent O2 diffusion dominates even under resting conditions.
What then is the function of Mb? Recent studies have claimed an NO scavenging role, even though other experiments have not detected the required presence of oxidized Mb (Flogel et al. 2001; Kreutzer & Jue, 2006; Kreutzer & Jue, 2004). Another perspective emerges from skeletal muscle experiments. At the start of muscle contraction, Mb desaturates within 30s to reach a steady state deoxygenation state, even though the cell has abundant O2 to drive oxidative phosphorylation (Mole et al. 1999; Chung et al. 2005). MbO2 appears to respond to a transient rather than a steady state change in energy demand.
The Mb translational diffusion coefficient also yields insight into the cellular environment. In solution, Mb diffuses isotropically; in the cell, Mb can exhibit anisotropic diffusion, since the longitudinal dimension exceeds the radial dimension by a factor of 10. However, the translational diffusion measurements show no orientation preference. Consequently, within a mean squared displacement of 3.8 µm, as determined from the measured DMb, Mb encounters no diffusion restrictions and experiences no cellular crowding, which could potentially modulate chemical reactivity. The cell contains many local fluid domains.
In summary, pulsed-field gradient NMR methods have determined the endogenous Mb diffusion in respiring rat myocardium. In myocardium, Mb cannot play a significant role to facilitate O2 diffusion. In skeletal muscle, it may play a role at the initiation of contraction. In marine mammalian muscle, Mb contribution to O2 flux predominates in the cell, which contains many local fluid domains.
What then is the function of Mb? Despite textbook reassurances, that simple question still challenges physiology and the search for the laws governing protein structure function relationship.
Acknowledgements
We gratefully acknowledge funding support from NIH GM 58688 (TJ), Philip Morris 005510 (TJ), and American Heart Association Western States Affiliate 0265319Y (UK) and the invaluable technical assistance of Jeff Walton and Jeff de Ropp.
References
Chung Y, Mole PA, Sailasuta N, Tran TK, Hurd R & Jue T (2005). Control of respiration and bioenergetics during muscle contraction. Am J Physiol Cell Physiol 288, C730–C738.
Flogel U, Merx MW, Godecke A, Decking UKM & Schrader J (2001). Myoglobin: a scavenger of bioactive NO. Proc Natl Acad Sci 98, 735–740.
Kreutzer U & Jue T (2006). Investigation of bioactive NO-scavenging role of myoglobin in myocardium. Eur J Physiol 452, 36–42.
Kreutzer U & Jue T (2004). The role of myoglobin as a scavenger of cellular NO in myocardium. Am J Physiol 286, H985–H991.
Kreutzer U, Mekhamer Y, Chung Y & Jue T (2001). Oxygen supply and oxidative phosphorylation limitation in rat myocardium in situ. Am J Physiol Heart Circ Physiol 280, H2030–H2037.
Kreutzer U, Wang DS & Jue T (1992). Observing the 1H NMR signal of the myoglobin Val-E11 in myocardium: An index of cellular oxygenation. Proc Natl Acad Sci 89, 4731–4733.
Mole PA, Chung Y, Tran TK, Sailasuta N, Hurd R & Jue T (1999). Myoglobin desaturation with exercise intensity in human gastrocnemius muscle. Am J Physiol 277, R173–R180.
Stejskal EO & Tanner JE (1965). Spin diffusion measurements: spin echoes in the presence of a time-dependent field gradient. J Chem Phys 42, 288–292.
Wang D, Kreutzer U, Chung Y & Jue T (1997). Myoglobin and hemoglobin rotational diffusion in the cell. Biophys J 73, 2764–2770.
Zhang J, Murakami Y, Zhang Y, Cho Y, Ye Y, Gong G, Bache R, Ugurbil K & From AHL (1999). Oxygen delivery does not limit cardiac performance during high work states. Am J Physiol 276, H50–H57.