
Physiology News Magazine
Control of upper airway muscles and the pathophysiology of obstructive sleep apnoea
OSA is more than just a loud case of snoring - it has been described as ‘the most important new disease discovered in the second half of the 20th century’. But why do airways close during sleep but not when we are awake? Robert Fogel offers an explanation
Features
Control of upper airway muscles and the pathophysiology of obstructive sleep apnoea
OSA is more than just a loud case of snoring - it has been described as ‘the most important new disease discovered in the second half of the 20th century’. But why do airways close during sleep but not when we are awake? Robert Fogel offers an explanation
Features
Robert B. Fogel
Instructor in Medicine, Harvard Medical School & Associate Physician, Division of Sleep Medicine, Brigham and Women’s Hospital, Boston, MA, USA
https://doi.org/10.36866/pn.54.26

Obstructive sleep apnoea (OSA) is a common disorder, affecting approximately 2% to 4% of middle-aged women and men in the United States (Young et al. 1993). This disorder is characterised by recurrent sleep-induced collapse of the pharyngeal airway, leading to hypoxaemia and hypercapnia (elevated blood CO2 levels), with arousal from sleep being required to re-establish airway patency. While the pathophysiology of this disorder is complex, and incompletely understood, most evidence supports the concept that patients with OSA have an anatomical predisposition to airway collapse (Schwab et al. 1995). However, during wakefulness protective mechanisms maintain pharyngeal airway patency by increasing the activity of pharyngeal dilator muscles. These protective mechanisms fail during sleep, with subsequent collapse of the pharyngeal airway behind the palate, tongue or both. Thus understanding the mechanisms controlling the human upper airway muscles is paramount to understanding the disorder. This article reviews some of the recent data regarding control of upper airway muscles in humans during wakefulness and sleep, as it applies to the pathogenesis of OSA.
The pharyngeal airway is a complex structure whose functions include respiration, speech, and swallowing. It is hypothesised that the evolution of speech in man, which requires substantial mobility of the larynx, led to the loss of rigid support of the hyoid bone which is present in most mammals. The human pharyngeal airway is thus largely dependent on muscle activity to maintain patency.
The muscles of primary importance fall into three groups:
(i) the muscles influencing hyoid bone position;
(ii) the muscle of the tongue
(genioglossus); and
(iii) the muscles of the palate.
The activity of many of these muscles is increased during inspiration, and this acts to counterbalance the collapsing influence of negative airway pressure. These muscles are referred to as inspiratory phasic upper airway muscles with the genioglossus being the best studied. Other muscles such as the tensor palatini do not demonstrate inspiratory phasic activity, but instead maintain a relatively constant level of activity. These are called tonic, or postural muscles and are also thought to play a role in the maintenance of airway patency. These two types of pharyngeal muscles are likely controlled by groups of neurones within the brainstem that have different firing patterns relative to the respiratory cycle and may behave quite differently during wakefulness and sleep.
It is known that the activity of the genioglossus is carefully controlled by a number of variables (Fig. 1) including:
(i) central pattern generating neuron output from the ventrolateral medulla;
(ii) respiratory chemostimulation via rising pCO2 or falling pO2;
(iii) a ‘wakefulness drive’ that may work through state-dependent systems outlined below.
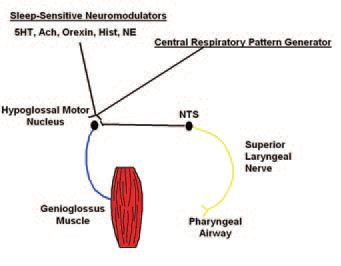
Finally, it appears that negative pressure in the pharynx is the most important local stimulus to genioglossal muscle activation on a moment by moment basis. Either a rapid pulse of negative pressure applied to the pharyngeal airway, or the application of inspiratory resistive load leads to an immediate and robust activation of this muscle in humans, and the increase in genioglossus muscle EMG (GGEMG) is proportional to the size of the load (Horner, 1991; Malhotra et al. 2000). In addition, if humans are ventilated using an iron-lung (negative pressure ventilator), when central pattern generating input can be largely abolished (as measured by an absence of phasic diaphragm EMG), there remains a linear relationship between pharyngeal negative pressure and genioglossal EMG (Fogel et al. 2001). This relationship remains constant over a range of negative pressures and is not affected by alterations in CO2 levels. This system thus provides a mechanism by which the pharyngeal muscles can respond on a moment by moment basis to changes in local pharyngeal conditions.
In the patient with OSA, in the face of a smaller and more collapsible upper airway, genioglossal and tensor palatini muscle activity have been demonstrated to be higher during wakefulness than in healthy individuals, probably secondary to the reflex mechanisms outlined above. The application of nasal continuous positive airway pressure (which decreases negative pressure fluctuations in the pharynx), decreases GGEMG to a much greater extent in apnoea patients, suggesting augmented activation of this negative pressure reflex in these patients. In addition, the tonic (expiratory) GGEMG is higher in the apnoea patient than in controls. While increased negative pressure is likely driving the increased phasic activity of the genioglossus, what controls tonic activation of this muscle is poorly understood.
The effects of sleep on pharyngeal muscle function are quite profound as well. In normal individuals, GGEMG falls slightly at sleep onset, but then recovers to near waking levels in the face of increased pharyngeal resistance. When placed on nasal continuous positive airway pressure, the initial sleep-induced decrease in GGEMG is not altered, suggesting that this initial fall is due solely to the removal of a ‘wakefulness’ drive in normal individuals.
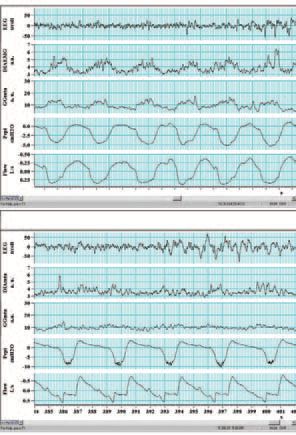
Sleep is also a state that is associated with a significant reduction in multiple neural reflex mechanisms, including postural, spindle-driven reflexes. This appears to be the case for negative pressure reflexes in the upper airway as well. Studies suggest that during non-rapid eye movement (non-REM) sleep this negative pressure reflex is substantially diminished (Wheatley et al. 1993). Furthermore, the relationship between changes in epiglottic pressure and GGEMG is much lower during sleep. During passive negative pressure ventilation in the iron-lung, there is a marked reduction in the GGEMG response to negative pressure during non-REM sleep compared to wakefulness, leading to a rise in airflow resistance, and in some normal subjects the development of airway obstruction (Fig. 2) (Fogel et al. 2003). The state-dependent neural mechanisms responsible for the loss of these neural reflex mechanisms is unclear at this time. However, virtually all neurotransmitter systems driving wakefulness (serotonin, noradrenaline, acetylcholine, histamine and orexin) project monosynaptically to the hypoglossal motor nucleus (controlling the genioglossus) and all are excitatory. Thus, when these neural systems become less active during sleep, it is not surprising that muscle activity falls.
From the data reviewed above, it would seem that the primary defect in obstructive sleep apnoea is an anatomically small or collapsible pharyngeal airway. During wakefulness, neuromuscular compensatory systems function to increase the activity of the pharyngeal dilator muscles, thus preserving airway patency.
However, this reflex-driven augmented muscle activity is lost at sleep onset, and collapse of the pharyngeal airway occurs. The associated hypoxaemia and hypercapnia drive increase respiratory effort and, ultimately, arousal from sleep occurs, thus re-establishing airway patency and ventilation. Once the patient returns to sleep, the cycle begins again. The patient thus suffers the consequences of repeated sleep disruption as well as recurrent hypoxaemia and hypercapnia.
As we learn more about the basic neural pathways that control upper airway muscle activity during wakefulness and sleep, the hope is that more specific therapy for this important disorder can be developed.

References
Fogel R, Malhotra A, Pillar G, Edwards J, Beauregard J, Shea S & White D (2001). Genioglossal activation in patients with obstructive sleep apnea versus control subjects. Mechanisms of muscle control. Am . Resp Cri. Care Med 164, 2025-2030.
Fogel RB, Trinder J, Malhotra A, Stanchina M, Edwards JK, Schory KE & White DP (2003). Within-breath control of genioglossal muscle activation in humans: effect of sleep-wake state. J Physiol 550, 899-910.
Horner RL, Innes, JA, Murphy K & Guz A (1991). Evidence for reflex upper airway dilator muscle activation by sudden negative airway pressure in man. J Physiol 436, 15-29.
Malhotra A, Pillar G, Fogel R, Beauregard J & White D (2000). Genioglossal but not palatal muscle activity relates closely to pharyngeal pressure. Am J Resp Crit Care Med 162, 1058-1062.
Schwab RJ, Gupta KB, Gefter WB, Metzger LJ, Hoffman EA & Pack AI (1995). Upper airway and soft tissue anatomy in normal subjects and patients with sleep-disordered breathing. Significance of the lateral pharyngeal walls. Am J Resp Crit Care Med 152, 1673-1689.
Wheatley J, Mezzanotte W, Tangel D & White D (1993). Influence of sleep on genioglossus muscle activation by negative pressure in normal men. Am Rev Respir Dis 148, 597-605.
YoungT, Palta M, Dempsey J, Skatrud J, Weber S & Badr S (1993). The occurrence of sleep-disordered breathing among middle-aged adults. New Engl J Med 32, 1230-1235.
