
Physiology News Magazine
Cortical control of human jaw muscles: bilateral but not symmetrical
Transcranial magnetic stimulation is revealing new details of the cortical control of human jaw muscles
Features
Cortical control of human jaw muscles: bilateral but not symmetrical
Transcranial magnetic stimulation is revealing new details of the cortical control of human jaw muscles
Features
Sophie L Pearce & Michael A Nordstrom
Discipline of Physiology, School of Molecular & Biomedical Science University of Adelaide, Australia
https://doi.org/10.36866/pn.54.16
Movements of the jaw during everyday activities such as chewing and speech require the bilateral co-ordination of muscles acting on the mandible. Control of these movements needs to be extremely precise to bring the teeth reliably into contact on each chewing stroke (with sub-millimetre tolerances), and to avoid damaging vulnerable oral structures such as the tongue and cheeks. Three muscles innervated by the trigeminal nerve control jaw closing and two main muscles control jaw opening on each side. Although the muscles on each side are usually activated together, the jaw-closers such as masseter can be controlled relatively independently when the situation demands (Butler et al. 2001). While voluntary control of the jaw muscles is mediated by the motor cortex, there are differences with the more widely studied limb muscles. For example, while fast, monosynaptic pathways from the motor cortex to motoneurons (corticomotoneuronal, or CM cells) are known to be involved in the precise, selective control of hand muscles, the evidence for the existence of such cells in the trigeminal system has been largely indirect. Furthermore, motoneurons of hand muscles receive a corticospinal projection almost exclusively from the contralateral hemisphere, while corticobulbar neurons from each hemisphere project to the vicinity of the trigeminal motor nuclei on both sides (Kuypers, 1958).
This arrangement poses several questions. What contribution does this bilateral cortical control system make to activation of jaw muscles? If CM cells exist in this bilateral system, how are they organised?
Figure 1 illustrates several possibilities for the cortical control of masseter motoneurones. While there is indirect evidence that axons of single CM cells branch to innervate jaw muscle motoneurons on both sides of the body (Carr et al. 1994), these neurones (green in Fig. 1) could not directly mediate selective activation of left and right muscles. Most textbooks describe the corticobulbar projections as bilateral and symmetrical, implying that each hemisphere makes a comparable contribution to control of the jaw muscles. The dual innervation undoubtedly helps to preserve control of masticatory muscles following unilateral stroke (Cruccu et al. 1988), but do the two hemispheres play distinct roles in controlling the jaw muscles on each side of the body?

These questions can be investigated in humans using a technique known as transcranial magnetic stimulation (TMS), in which a brief magnetic field is induced by a stimulating coil placed on the scalp over the motor cortex. This painless, non-invasive technique activates corticospinal or corticobulbar neurons within the motor cortex, which in turn elicit a response in the muscles of interest that can be detected using electromyography (EMG).
Early studies using surface EMG and TMS reported fast-conducting excitatory projections from the motor cortex to the masseter motoneuron pools on both sides: however, the circular coil used in these studies may have activated both hemispheres simultaneously (Cruccu et al. 1989). When we confined the stimulus to one hemisphere by using a figure-8 TMS coil, the response evoked in masseter was still bilateral, and was ~40% larger in the contralateral muscle (Butler et al. 2001). The response latency on each side is ~7 ms, which is consistent with a CM projection to both muscles from one hemisphere. We also showed that selective activation of the masseter muscle on one side during a unilateral bite was mediated by corticobulbar neurons in the contralateral but not the ipsilateral hemisphere (Butler et al. 2001). Clearly the two hemispheres have distinct roles in this task.
We suggest that selective activation of one masseter is accompanied by reduced excitation of CM cells in the contralateral hemisphere which branch to innervate both masseter motoneuron pools (the green projection in Fig. 1). The larger contralateral responses to focal TMS and their task-dependence point to a second population of CM cells with exclusively contralateral projections to masseter motoneurons (blue projection in Fig. 1). We found no evidence for the existence of the red CM cells.
With surface EMG studies such as these, the evidence for monosynaptic excitation of trigeminal motoneurons by CM cells is strong, but not conclusive, as it is based on latency measures and assumptions about conduction velocities of the axons involved. To provide unambiguous evidence of CM projections to motoneurons it is necessary to record responses of single motor units to TMS. A motor unit consists of a single motoneuron, its axon and the skeletal muscle fibres it innervates. Motor unit activity can be detected in humans by fine-wire electrodes inserted into the muscle. Because of the secure 1:1 transmission of the action potential between nerve and muscle, this technique gives information about the discharge pattern of a single motoneuron in the brainstem (in the case of the jaw muscles).
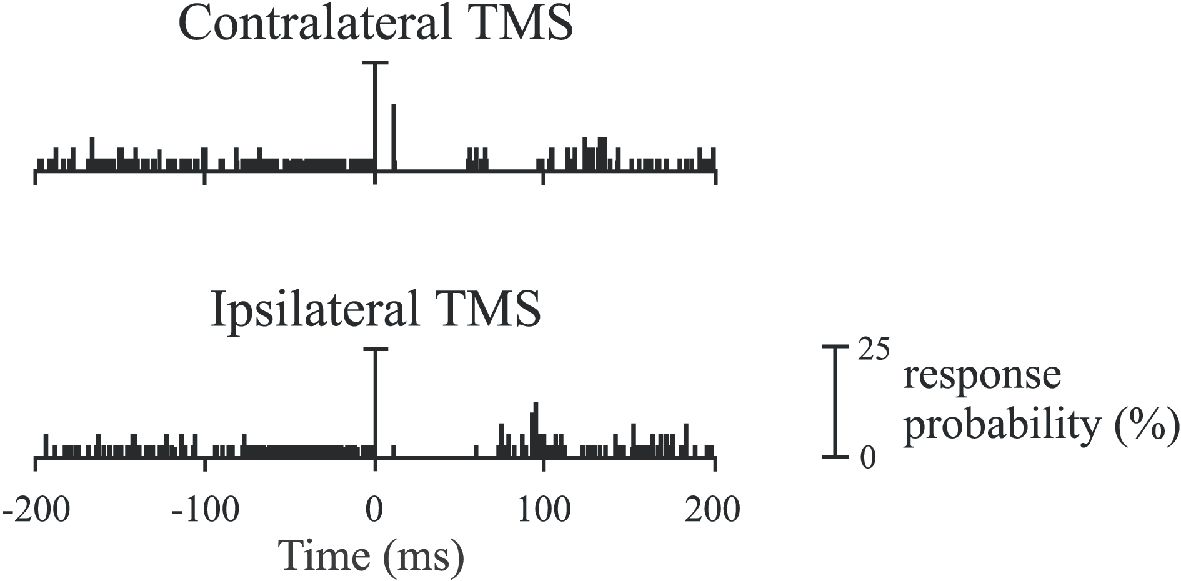
Single motor unit recordings from masseter and digastric muscles during weak voluntary contractions confirm the existence of CM projections to trigeminal motoneurons (Gooden et al. 1999; Pearce et al. 2003), and show that single motor units are differentially controlled by the two hemispheres. An example of the responses of a masseter motor unit to focal TMS of the contralateral and ipsilateral hemisphere is shown in Fig. 2. As in this example, most of the masseter motor units were excited at monosynaptic latency only by TMS of the contralateral hemisphere. A minority (25%) were excited at monosynaptic latency by TMS of either hemisphere. The excitatory response to contralateral and ipsilateral TMS (when present) was very brief (~1.5 ms), and consistent with production of a monosynaptic excitatory post-synaptic potential in the masseter motoneuron by the corticobulbar neurons activated by TMS. This narrow peak is conclusive proof for CM cell projections to masseter. At low biting forces, most of the direct excitation of masseter comes from the contralateral motor cortex. Many masseter motor units active at low forces do not receive excitatory inputs from the ipsilateral hemisphere. This would contribute to the smaller response to TMS seen in the ipsilateral muscle with surface EMG. In contrast, most digastric motor units receive excitatory inputs from both hemispheres (Gooden et al. 1999). These differences presumably contribute to the more independent activation of the masseter muscles on each side during functional tasks than the symmetrical pattern observed for the digastric muscles.
The functional organization of the corticobulbar projection to the trigeminally innervated jaw muscles is complex. The traditional model, based on anatomical evidence, of bilateral and symmetrical corticobulbar projections is no longer tenable. We have demonstrated that motor cortices of both hemispheres are involved in the control of trigeminal motoneurons, although the contralateral hemisphere has a greater effect overall, particularly at low forces. The two hemispheres play distinct roles in the control of ipsi-and contralateral muscles, and this differs for jaw-closers and openers. The cortical control of all motor units in a muscle is not uniform. Some motor units are excited exclusively from the contralateral hemisphere, while others receive bilateral excitatory inputs. This complexity in the corticobulbar innervation of jaw muscles may assist controlled biting on one side when food is held between the teeth. These observations suggest that functional deficits in masticatory muscles following unilateral stroke should be greater for muscles contralateral to the lesion. This has been shown for maximal voluntary activation of masseter (Cruccu et al. 1988), but more subtle deficits in fine control of the contralateral jaw muscles would also be expected, although these have not been explored in patients.
References
Butler SL, Miles TS, Thompson PD & Nordstrom MA (2001). Task-dependent control of human masseter muscles from ipsilateral and contralateral motor cortex. Exp Brain Res 137, 65-70.
Carr LJ, Harrison LM & Stephens JA (1994). Evidence for bilateral innervation of certain homologous motoneurone pools in man. J Physiol 475, 217-227.
Cruccu G, Berardelli A, Inghilleri M & Manfredi M (1989). Functional organization of the trigeminal motor system in man. A neurophysiological study. Brain 112, 1333-1350.
Cruccu G, Fornarelli M & Manfredi M (1988). Impairment of masticatory function in hemiplegia. Neurology 38, 301-306.
Gooden BR, Ridding MC, Miles TS, Nordstrom MA & Thompson PD (1999). Bilateral cortical control of the human anterior digastric muscles. Exp Brain Res 129, 582-591.
Kuypers HGJM (1958). Corticobulbar connexions to the pons and lower brain-stem in man. Brain 81, 364-388.
Pearce SL, Miles TS, Thompson PD & Nordstrom MA (2003). Responses of single motor units in human masseter to transcranial magnetic stimulation of either hemisphere. J Physiol 549, 583-596.
