
Physiology News Magazine
Coupling or not coupling of mitochondria to Ca2+ sources in neurones. Soma and neurites differ
Bioluminescence imaging of aequorin targeted to mitochondria shows that these organelles are closely coupled to the endoplasmic reticulum in the soma but not in the neurites of sympathetic neurones. This functional organization could contribute to generate spatial differences in neuronal Ca2+ signals
Features
Coupling or not coupling of mitochondria to Ca2+ sources in neurones. Soma and neurites differ
Bioluminescence imaging of aequorin targeted to mitochondria shows that these organelles are closely coupled to the endoplasmic reticulum in the soma but not in the neurites of sympathetic neurones. This functional organization could contribute to generate spatial differences in neuronal Ca2+ signals
Features
Lucía Núñez, Carlos Villalobos, & Javier García-Sancho
Instituto de Biología y Genética Molecular, University of Valladolid and Spanish Research Council, Valladolid, Spain.
https://doi.org/10.36866/pn.70.23

Ca2+ signals play a pivotal role in neuronal functions including excitability, secretion, learning and memory. They are triggered by the opening of plasma membrane, voltage-operated Ca2+ channels (VOCCs) and amplified by Ca2+-induced Ca2+ release (CICR) from the endoplasmic reticulum (ER). It is becoming evident that, in addition to their role as cell powerhouses and central players in the intrinsic pathway for apoptosis, mitochondria are essential for modulation of Ca2+ signals. However, research on mitochondrial Ca2+ homeostasis has been hampered by technical constraints and the difficulty for accurate monitoring of mitochondrial Ca2+, particularly in single neurons. The introduction of protein Ca2+ probes targeted to mitochondria has paved the way for better understanding of mitochondrial Ca2+ signalling (Rizzuto et al. 1998).
Mitochondria can take up Ca2+ through the mitochondrial Ca2+ uniporter, a low-affinity, high capacity system that permits Ca2+ uptake towards the huge electrochemical gradient built at the inner mitochondrial membrane by the respiratory chain. As Ca2+ uptake by mitochondria requires relatively high cytosolic Ca2+, it was believed for a long time that mitochondria did not play a role in Ca2+ signalling under physiological conditions, being only able to buffer large, pathological Ca2+ overloads. However, recent evidence has shown that mitochondrial Ca2+ influx follows almost always normal, ‘physiological’, cytosolic Ca2+ increases. To explain these findings it has been proposed that high Ca2+ microdomains are transiently generated near mitochondria close enough to the mouth of the Ca2+ channels at sites of Ca2+ entry or release. The Ca2+ concentration at these hot spots would be high enough to activate rapid Ca2+ uptake through the uniporter in surrounding mitochondria. For example, mitochondria in close contact with ER take up Ca2+ from high Ca2+ microdomains in the mouth of the Ca2+ release channels opened by IP3 (Rizzuto et al. 1998).
Aequorins with low affinity for Ca2+ were initially developed for monitoring high Ca2+ levels inside the ER, but their targeting to mitochondria revealed two important new concepts in mitochondrial Ca2+ homeostasis (Montero et al. 2000). First, that only a pool of mitochondria, the one close enough to sites of Ca2+ entry and release, is able to ‘sense’ the high Ca2+ microdomains. This mitochondrial pool takes up Ca2+ avidly, whereas the remaining mitochondria do not see the Ca2+ hot spots and take up much less Ca2+. Second, that the increase in mitochondrial Ca2+ in the sensitive mitochondrial pool may reach as much as 10-3 M, a value 100 fold larger than previous estimates. In fact, a quantitative estimation of the Ca2+ redistribution after stimulation of chromaffin cells suggested that most of the Ca2+ entering cells through VOCCs is actually cleared from the cytosol by the surrounding mitochondria rather than by other extrusion systems (Villalobos et al. 2002). In chromaffin cells, the same mitochondrial pool is functionally coupled to VOCCs and ER. This organization may help to avoid spreading of entering Ca2+ towards the cell core and to tune up local ATP synthesis to power exocytosis.
Ca2+ signalling in neurones can be even more complex. It has been proposed that distinct intracellular Ca2+ transients in neurites and somata may integrate neuronal signals (Johenning et al. 2002). The mechanisms underlying the generation of spatially specific Ca2+ signals is not well understood. Mitochondria modulate cytosolic Ca2+ signalling in neurons by buffering the Ca2+ rises evoked by Ca2+ entry or Ca2+ release. This was indirectly suggested by the increase in the cytosolic Ca2+ responses after preventing mitochondrial Ca2+ uptake with protonophores in sensory neurons (Shishkin et al. 2002). The low level of bioluminescent light emission by aequorin has hampered imaging of mitochondrial Ca2+ in individual neurons for a long time. However, combining the use of ultra-sensitive photon counting cameras with high-efficient protein expression systems has enabled recently imaging of individual neurons expressing targeted aequorin (Núñez et al. 2007). Fig. 1 shows the photonic emissions imaged from sympathetic neurons expressing aequorin targeted to mitochondria. The neurons were stimulated with either high-K+ solution, which depolarises the plasma membrane and triggers Ca2+ entry trough VOCCs, or with caffeine, which stimulates Ca2+ release from the ER. Both stimuli triggered photonic emissions, which reflects the mitochondrial Ca2+ uptake induced by Ca2+ entry or Ca2+ release. Only a subpopulation (30–40%) of mitochondria ‘sensed’ these stimuli, suggesting the existence of two different pools of mitochondria, one functionally coupled and the other not coupled to the different Ca2+ sources.
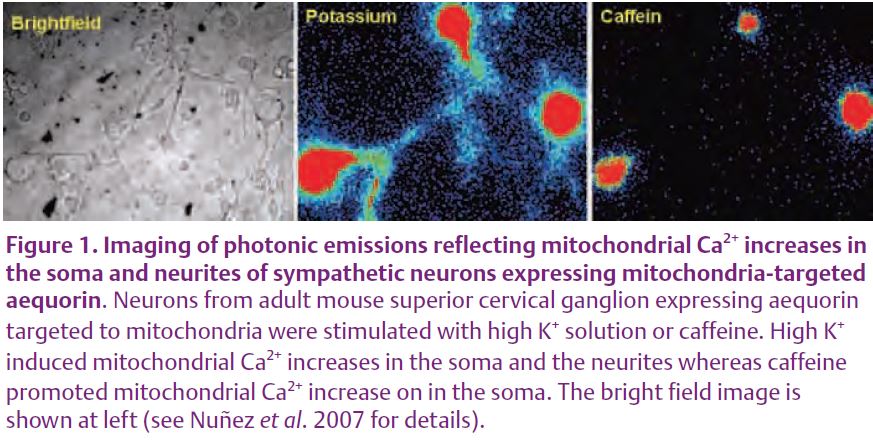
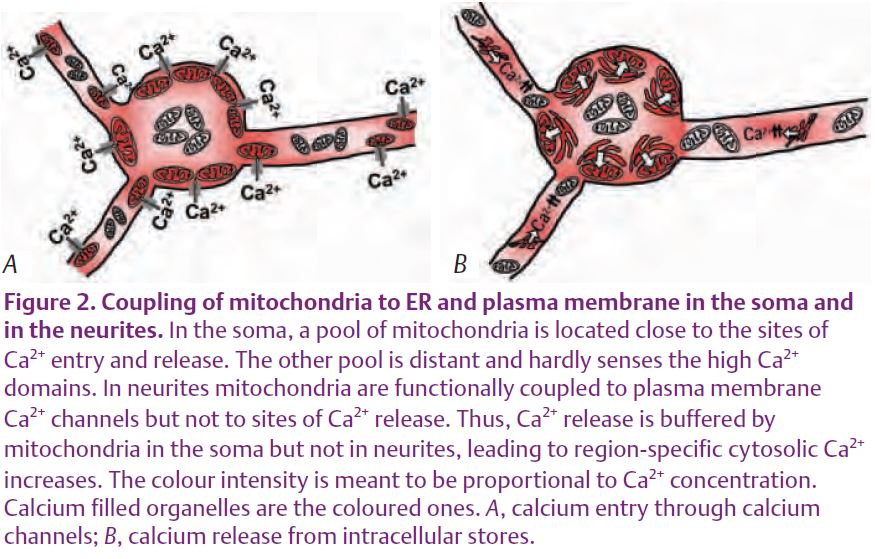
Interestingly, mitochondria at soma and neurites behaved differently. Whereas mitochondria in the soma were stimulated by caffeine, mitochondria at neurites were not. These differences were not seen when the neurons were stimulated with high K+ (Fig. 1). As a consequence of the lack of mitochondrial buffering, the cytosolic Ca2+ rise induced by caffeine was larger in the neurites than in the soma (Núñez et al. 2007). These results agree with previous reports where mitochondria seemed to buffer Ca2+ entry but not Ca2+ release in dorsal root ganglion neurons (Svichar et al. 1997). In summary, these results suggest that strategic location of mitochondria in different regions of the neuron may contribute to the generation of spatially specific Ca2+ signals (Fig. 2). For example, direct measurements in cell bodies and in the proximal and distal dendrites of cerebellar Purkinje neurons showed that amplification due to CICR is more efficient as the dendritic branches become thinner. Whether local mitochondria take up Ca2+ or not may have important physiological consequences. Apart from the buffering of the cytosolic Ca2+ signal, the increase in mitochondrial Ca2+ promotes local ATP synthesis that may be required for exocytosis. In addition, Ca2+ taken up by mitochondria is released later back to the cytosol. This delayed enhancement of cytosolic Ca2+ has been suggested to be involved in post-tetanic potentiation. Thus, coupling or not coupling of mitochondria to the different Ca2+ sources may lead to region-specific modifications of Ca2+ signals that may underlie important neuronal functions.
References
Johenning FW, Zochowski M, Conway SJ, Holmes AB, Koulen P & Ehrlich BE (2002). Distinct intracellular calcium transients in neurites and somata integrate neuronal signals. J Neurosci 22, 5344–5353.
Montero M, Alonso MT, Carnicero E, Cuchillo-Ibañez I, Albillos A, García AG, García-Sancho J & Alvarez J (2000). Chromaffin-cell stimulation triggers fast millimolar mitochondrial Ca2+ transients that modulate secretion. Nat Cell Biol 2, 57–61.
Núñez L, Senovilla L, Sanz-Blasco S, Chamero P, Alonso MT, Villalobos C & García-Sancho J (2007). Bioluminescence imaging of mitochondrial Ca2+ dynamics in soma and neurites of individual adult mouse sympathetic neurons. J Physiol 580, 385–395.
Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, Tuft RA & Pozzan T (1998). Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science 280, 1763–1766.
Shishkin V, Potapenko E, Kostyuk E, Girnyk O, Voitenko N & Kostyuk P (2002). Role of mitochondria in intracellular calcium signalling in primary and secondary sensory neurones of rats. Cell Calcium 32, 121–130.
Svichar N, Kostyuk P & Verkhratsky A (1997). Mitochondria buffer Ca2+ entry but not intracellular Ca2+ release in mouse DRG neurons. Neuroreport 8, 3929–3932.
Villalobos C, Núñez L, Montero M, García AG, Alonso MT, Chamero P, Alvarez J & García-Sancho J (2002). Redistributión of Ca2+ among cytosol and organella during stimulation of bovine chromaffin cells. FASEB J 16, 343–353.
