Physiology News Magazine
Cystic fibrosis: a lesson in chemistry
Paul Quinton and Ruth Muchekehu examine a (micromolecular) sticky problem
Features
Cystic fibrosis: a lesson in chemistry
Paul Quinton and Ruth Muchekehu examine a (micromolecular) sticky problem
Features
Ruth W. Muchekehu (1) and Paul M. Quinton (1, 2)
1: Department of Pediatrics-0830, School of Medicine, University of California–San Diego, 9500 Gilman Drive, La Jolla, CA 92093-0831, USA
2: Division of Biomedical Sciences, University of California–Riverside, Riverside, CA 92507-0121, USA
https://doi.org/10.36866/pn.81.37


Physiology and medicine usually look to the hard sciences for instruction. However, the pathogenic mucus in the disease cystic fibrosis (CF) may be instructive for physics and chemistry in the forming of mucus gels. The expansion of mucins into mucus gels is largely understood in terms of the exchange of monovalent for divalent cations in anionic mucin networks (Verdugo, 1991). Something may be missing though, since the cause of thick, sticky mucus in CF patients still perplexes physiologists and biochemists more than 60 years after the first descriptions of the disease. Some clues may come from the abnormally thick mucus and defects in bicarbonate secretion (HCO3–) found in CF.
Mucin molecules, the main component of gel-forming mucus, are extremely long highly charged anionic polymers that are stored in mucus cell granules at 1/1000 of their final volume after secretion. To effect this tight, pre-secretory packing, the strong electrostatic repulsion of mucin negative charges is neutralized by protons and high concentrations of divalent Ca2+. Until now, the physical chemistry of mucin polymer expansion during secretion relied principally upon a Donnan effect thought to be due to simply exchanging these bound cations for mobile monovalent K+ and/or Na+ that almost instantly ‘hydrates’ the mucin molecules by about 1000-fold (Verdugo, 1991). However, the fluids that accompany mucus secretion
in CF are replete with Na+ and K+, and yet the mucus afflicting the many glands in CF remains thick and adhesive.
Currently, the thick mucus in CF is generally assumed to be due to an abnormal hyperabsorption of fluid from secreted mucins. For example, it is thought that water is excessively absorbed from mucus secreted onto the airway surface in CF lungs making it difficult to remove. However, many of the organs afflicted by thick mucus in CF do not have this capacity to reabsorb fluids. The facts thus dictate that something is still missing in our understanding of the physics and chemistry of mucus gel expansion. The disease pathophysiology implicates HCO3–.
Both mucus and bicarbonate equip the body with a protective gel that coats the surfaces of hollow organs, providing a physical barrier and chemical buffer that guard the epithelial surface. Mucus production must be delicately balanced; too little mucus leaves cells vulnerable to solid and chemical irritants as well as bacterial and viral pathogens, and too much mucus leads to stagnation, inflammation and infections. As the predominant extracellular buffer, bicarbonate (HCO3–) must support gastroduodenal defence, suspend bile, stabilize digestive pro-enzymes, impede bacterial binding and serve as a critical adjuvant to antibacterial defensins. It is perhaps no marvel that mucus and HCO3– are allies, but are they more intimately involved?
CF provides clues that intrinsically link mucus and HCO3–. CF is a hereditary disease caused by mutations in the gene that encodes the cystic fibrosis transmembrane conductance regulator (CFTR) Cl– channel protein, whose loss of function inhibits both Cl– and HCO3– transport. Concurrent losses of HCO3– secretion occur in tissues affected by abnormal mucus in CF, and the loss of HCO3– transport seems to correlate with severity of the CF pathology (Choi et al. 2001).
In the female reproductive tract, mucus viscosity and bicarbonate change inversely during the menstrual cycle. When HCO3– is at its maximum concentration at ovulation, mucus viscosity is minimal, and when HCO3– is lowest at the follicular phase, mucus viscosity is at its peak. HCO3– douching improves sperm penetration of cervical mucus by reducing the viscosity of cervical mucus. This suggests the coordinated changes in mucus and HCO3– are not coincidental, especially given that mucus thinning is absent in women with CF. All these observations imply that HCO3– attends mucus viscosity.
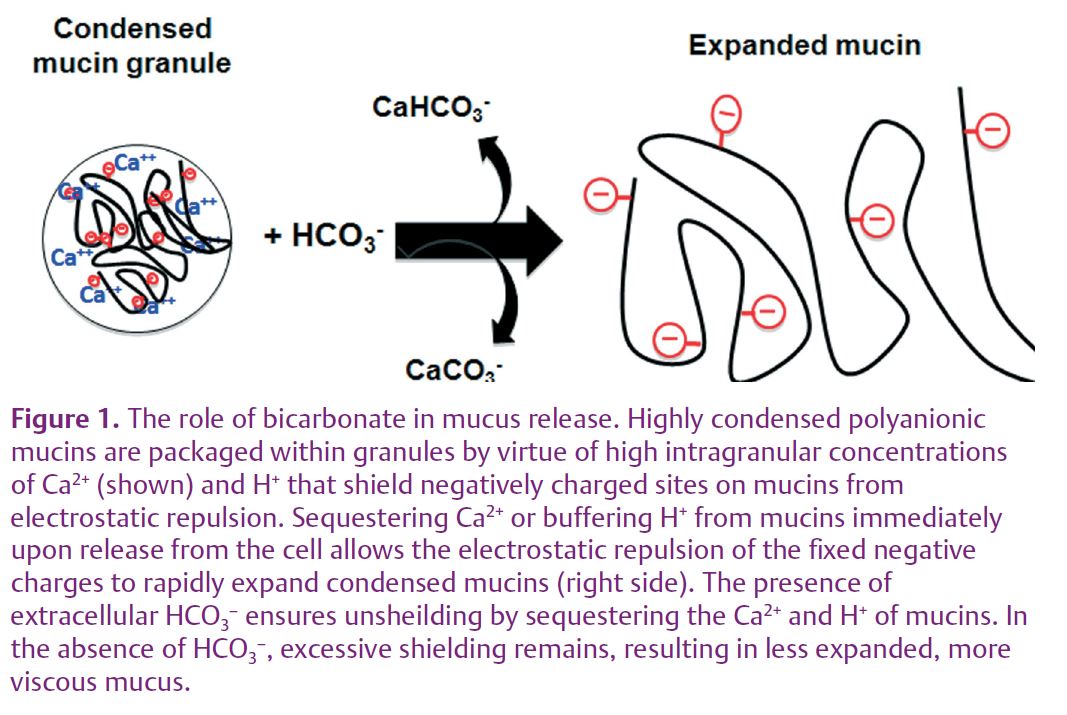
How, then, can HCO3– affect the physical properties of mucus? We surmise that, normally, secreted HCO3-– enables mucin expansion by sequestering the bound Ca2+ and H+ from secreted mucins. This will expose negative charges, enabling electrostatic forces to vigorously and rapidly open the mucin molecule (Fig. 1). That is, HCO3– changes the dynamics of mucin expansion by competing with the fixed anions of the mucin molecules for Ca2+ and H+ rather than Na+ and K+ competing with Ca2+ for the fixed anionic sites on mucins.
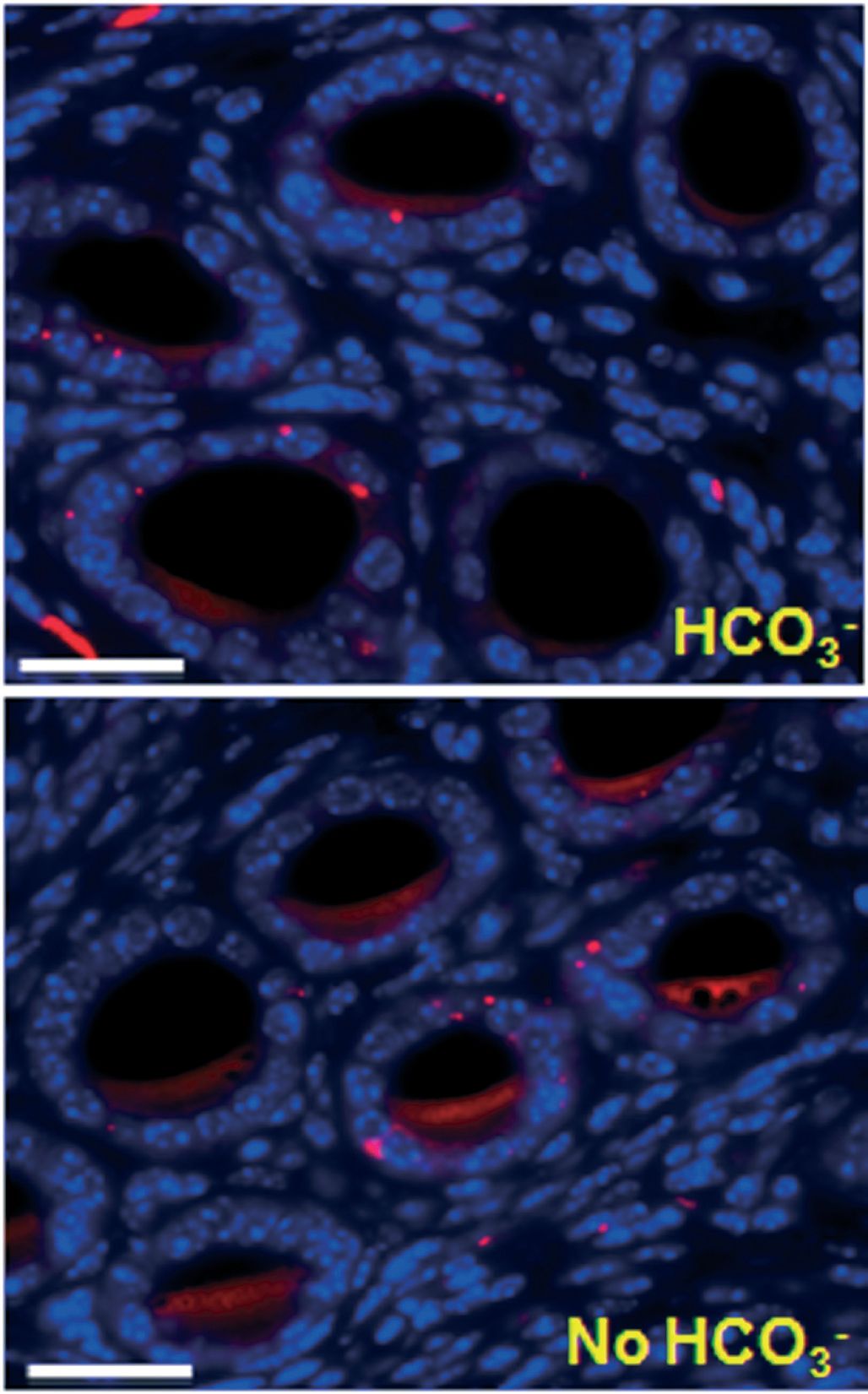
To test this notion, we used the female reproductive tract of wild type (WT) and CF ∆F508 mice mounted in a custom-fabricated perfusion chamber. The lumens were perfused and bathed basolaterally with either HCO3–-replete or HCO3–-depleted Ringer solution and stimulated to acutely release mucus into the luminal perfusate. In wild type mice, mucus release was severely impaired in the absence of extracellular HCO3– . In the CF ∆F508 homozygous reproductive tract, or when CFTR was inhibited pharmacologically in wild type tissues, mucus release was also significantly depressed (Muchekehu & Quinton, 2010).
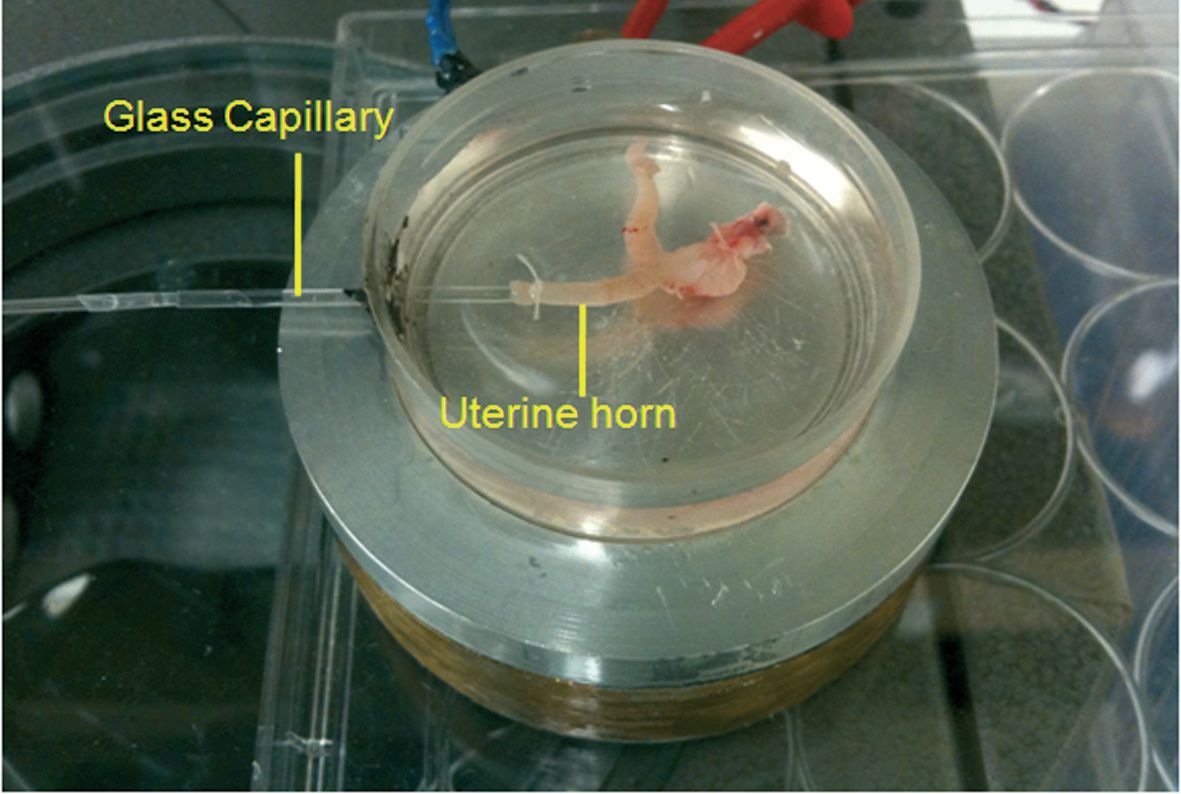
Cyclic adenosine monophosphate (cAMP)-mediated fluid secretion is depressed in CF and may be important for mucus discharge. We tested the importance of fluid secreted from the reproductive tract by canulating one horn of the closed uterine sac with a glass capillary (Fig. 2). Fluid secretion was monitored by the displacement of the air–liquid meniscus in the capillary. In contrast to mucus release, stimulated fluid secretion was not dependent on HCO3– or on CFTR function under these conditions. Moreover, without HCO3–, mucus remained ‘trapped’ in the lumens of the uterine glands (Fig. 3) (Muchekehu & Quinton, 2010).
These findings present a new role for HCO3– as a critical element in mucus release and in the regulation of mucus viscosity generally. Mucus thinning at the ovulatory stage of the menstrual cycle allows the passage of sperm through the reproductive tract, but this role is not limited to the reproductive tract as mucus release in the small intestine is also HCO3– dependent (Garcia et al. 2009). Moreover, the principal gel-forming mucin of the gut (MUC 2) is not the same as in the uterus (MUC 5B); and the viscosity of the third major gel-forming mucin (MUC 5AC) during stimulated secretion from cultured cells is markedly dependent on extracellular HCO3– levels (Chen et al. 2010). Adding to this, the common viscid mucus pathology affecting different mucins secreted by disparate organs in CF all strongly imply that HCO3-– is a common and necessary attendant to normal mucus expansion.
In a certain sense then, the tables of the sciences may be turned as the physiology of the female reproductive tract and the pathophysiology of CF offer new lessons for chemistry and physics on how mucin polymer gels expand.
References
Chen EY, Yang N, Quinton PM & Chin WC (2010). A new role for bicarbonate in mucus formation. Am J Physiol Lung Cell Mol Physiol 299, L542–L549.
Choi JY, Muallem D, Kiselyov K, Lee MG, Thomas PJ & Muallem S (2001). Aberrant CFTR-dependent HCO3– transport in mutations associated with cystic fibrosis. Nature 410, 94–97.
Garcia MA, Yang N & Quinton PM (2009). Normal mouse intestinal mucus release requires cystic fibrosis transmembrane regulator-dependent bicarbonate secretion. J Clin Invest 119, 2613–2622.
Muchekehu RW & Quinton PM (2010). A new role for bicarbonate secretion in cervico-uterine mucus release. J Physiol 588, 2329–2342. http://jp.physoc.org/content/588/13/2329.long
Verdugo P (1991). Mucin exocytosis. Am Rev Respir Dis 144, S33–S37.
Acknowledgements
This work was supported by grants from Cystic Fibrosis Research Inc., Nancy Olmsted Trust, Cystic Fibrosis Foundation, and NIH USPHS R01-HL084042.