Physiology News Magazine
Differential control of first and second pain by the midbrain
Pain is a plastic phenomenon, Simon McMullan investigates the differential effects of PAG on C-fibres and Aδ-fibres.
Features
Differential control of first and second pain by the midbrain
Pain is a plastic phenomenon, Simon McMullan investigates the differential effects of PAG on C-fibres and Aδ-fibres.
Features
Simon McMullan
Department of Physiology, University of Bristol
https://doi.org/10.36866/pn.46.11

Acute nociceptor activation usually drives behaviours that avoid the noxious stimulus as a result of the aversive nature of the pain signal. However, in certain circumstances survival is not enhanced if nociceptor-evoked activity overrides other motivational drives. A link between extreme emotional drive and inhibition of nociceptor evoked activity was made during the Second World War by Henry Beecher, a field surgeon. He observed that battle- injured soldiers requested analgesic drugs infrequently when compared to civilians who had received equivalent wounds in peacetime circumstances. He also realised that this ‘battlefield analgesia’ was temporary, and that normal sensitivities to trauma would return within hours. He concluded that the conscious and behavioural responses to painful stimuli were not hardwired, but were in fact dependent on the emotional state of the individual.
Since that time physiologists have identified brain regions that orchestrate defensive behaviours and associated autonomic and motor activities when stimulated in the conscious animal. Among these areas, the midbrain peri-aqueductal grey (PAG) is the most extensively studied, and is the focus of my research. When activated in behaving animals, the PAG elicits a range of defensive behaviours, from biting, hissing and arching of the back in the cat (Bandler & Carrive, 1988) to withdrawal, avoidance and aversion in the rat (Morgan & Carrive, 2001) Depaulis et al, 1994). These behaviours are supported by appropriate autonomic changes. The precise nature of the effects observed depends both on the region of the PAG stimulated and on the species. It is now evident that the coordinated defence response evoked from the PAG includes changes in sensory processing, more specifically, descending inhibition of spinal nociception. The resultant antinociception evoked by stimulation of the PAG is so profound that during stimulation through previously implanted electrodes surgical procedures can be performed on conscious animals without need of further analgesic intervention (Reynolds, DV, 1969).
Recent studies in this laboratory by Waters and Lumb (1997) demonstrated that, in most instances, descending control evoked from the PAG is indeed selective for the nociceptive responses of spinal dorsal horn neurones that are driven by noxious and by non-noxious stimulation in the periphery. However, there was a small population of dorsal horn neurones whose nociceptor evoked responses were unaffected or even enhanced following stimulation of the PAG. Nociceptive inputs to the dorsal horn are conveyed largely in two groups of primary afferent fibres: unmyelinated C-fibres and finely myelinated Aδ-fibres. Lamina V dorsal horn neurones can be divided into two groups on the basis of their inputs from C- and Aδ-fibres. Those neurones whose nociceptive input includes a C- fibre component (C+ve neurones) and those without a demonstrable C-fibre input, in which nociceptor evoked activity is presumed to be mediated by Aδ-fibres alone (C-ve neurones). Further investigation revealed that those dorsal horn neurones whose nociceptive responses were inhibited from the PAG were C+ve and those in which responses were unaffected or enhanced were C-ve.
C- and Aδ-nociceptors convey different qualities of the nociceptive message to the CNS (Torebjörk & Ochoa, 1990). As such, the consequences of the pattern of control evoked from the PAG are that the slowly conducted, poorly localised and arguably distracting component of the nociceptive message (second pain, conveyed in C-nociceptors) would be depressed, whereas the highly localised, rapidly conducted component (first pain, conveyed in Aδ-nociceptors) is left intact or even enhanced when the PAGis activated. This combination of effects would provide the animal with up to date information that could help direct motor activity and increase motivational drive whilst blocking those components of the nociceptive message that are redundant in an emergency situation.
The aim of my PhD was to develop a model by which I could further investigate the modulation of spinal nociception by the PAG, with particular focus on the differential control of C- and Aδ-nociception and its mechanisms. The first stage of this study was to find a way to preferentially activate the different groups of nociceptor. I modified a protocol devised by Yeomans and Proudfit (1996), using contact heating at different rates to preferentially activate Aδ- or C- cutaneous heat nociceptors. Application of fast (7.5°C/s) or slow (2.5°C/s) rates of heating to the dorsal surface of the hindpaw evoked withdrawal reflexes that were mediated preferentially by Aδ-fibres or C-fibres respectively. The thresholds and magnitudes of reflex responses were monitored by recording electromyographic (EMG) activity from the biceps femoris. Having established my model, a first series of experiments was designed to test whether injections of excitatory amino acid (DLH) into the PAG modulated withdrawals to fast and slow ramps in different ways. The results of these experiments revealed that 60% of withdrawals evoked by slow heating ramps are completely abolished, compared to only 30% of withdrawals evoked by fast ramps immediately after DLH injection into the PAG. Furthermore, in those instances in which withdrawals still occurred following PAG activation, thresholds to slow ramps were significantly increased, whereas thresholds to fast ramps were unchanged.
Therefore, using a direct approach I was able to conclude that neuronal activation in the PAG did indeed exert differential effects on C-fibre rather than Aδ-thermal nociceptor-evoked activity. I communicated these data at the Society’s meeting in Oxford in March last year (McMullan & Lumb, 2001).
DLH is non-selective, in that it will activate intrinsic interneurones in the PAG as well as output neurones. Output neurones involved in descending control of nociceptive processing are, at least in part, under tonic inhibitory GABAergic control. In my next series of experiments I compared the modulation of nociception evoked by non-selective neuronal activation in the PAG using DLH to that evoked by selective disinhibition of output neurones under tonic GABAergic control, using injections of the GABAA antagonist bicuculline into sites that had yielded pressor responses following DLH injection. Results of these experiments revealed a higher degree of differential control following disinhibition of PAG output neurones with bicuculline. This enhanced selectivity supports a role for this group of output neurones, usually under intrinsic inhibitory control. I communicated these data at a recent meeting of the Society in Bristol in September 2001 (McMullan & Lumb, 2001)
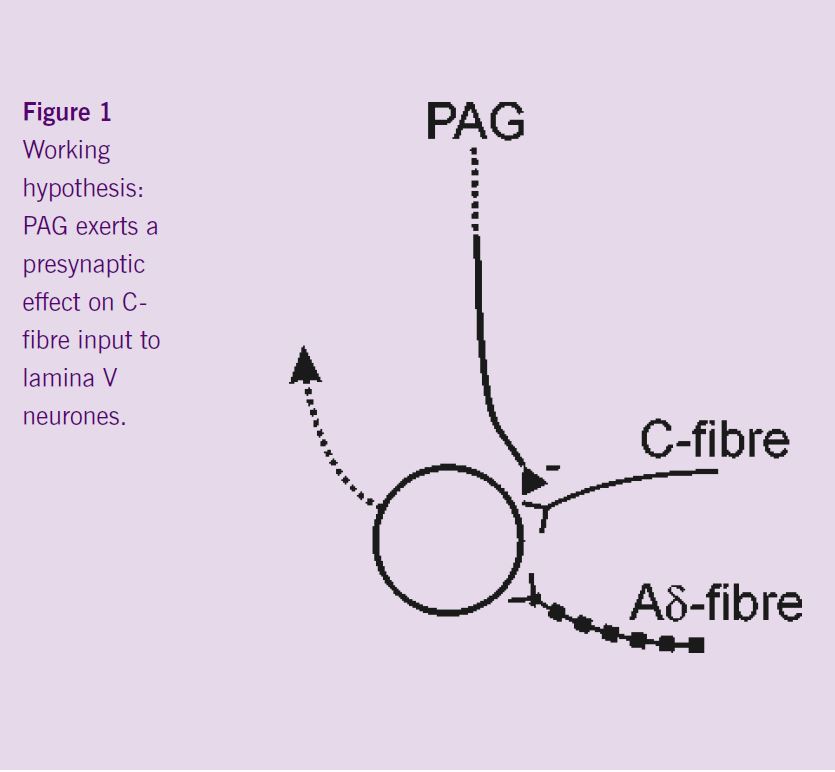
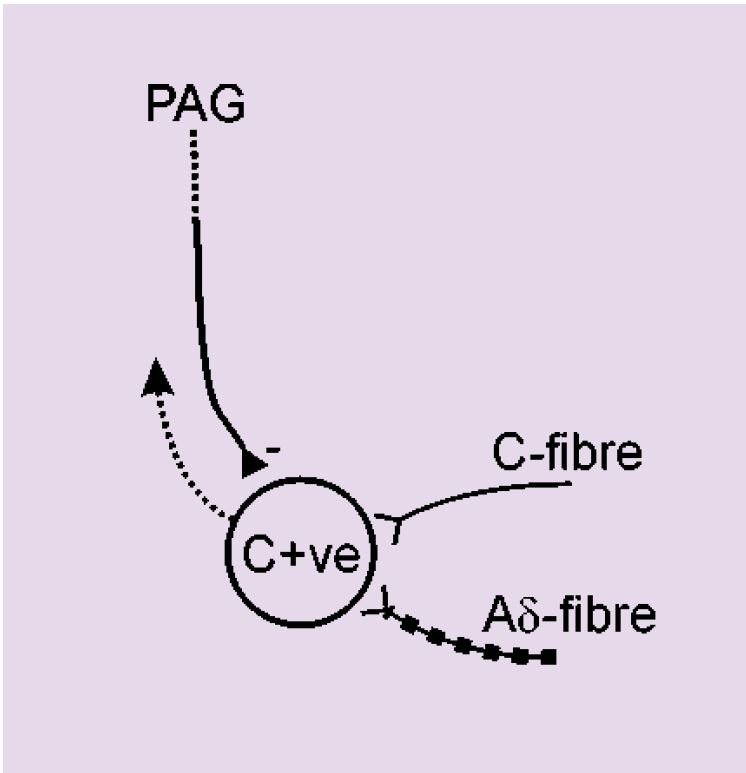
The next step of the project was a series of experiments that were designed to investigate spinal mechanisms by which the PAG could selectively inhibit C-fibre rather than Aδ-evoked responses. The hypothesis to be tested was that descending control from the PAG mediates its effects pre-synaptically to Lamina V dorsal horn neurones (Figure 1), such that C-fibre input to these cells would be inhibited by PAG activity, whereas input to these cells from Aδ-fibres would be preserved. Recordings were made from the lamina V C+ve neurones, i.e. neurones that received synaptic inputs from both Aδ- and C-fibres. The effect of DLH injection into the PAG was tested on the responses of these neurones to fast and slow rates of noxious heating in their peripheral receptive fields. Neuronal activation in the PAG inhibited the responses of these cells to both rates of noxious skin heating. These non-selective actions do not support the hypothesis that the PAG exerts its differential effects on C- and Aδ- responsiveness by a pre-synaptic modulation of C-fibre input to lamina V C+ve neurones. Interestingly, ongoing experiments have demonstrated a lack of inhibition of the responses of C-ve neurones to fast rates of skin heating. It would appear therefore that the differential effects evoked from the PAG might result from post-synaptic influences on the different dorsal horn neuronal cell types (C+ve vs. C-ve) rather than differential pre-synaptic modulation of inputs to all lamina V dorsal horn neurones (Figure 2).
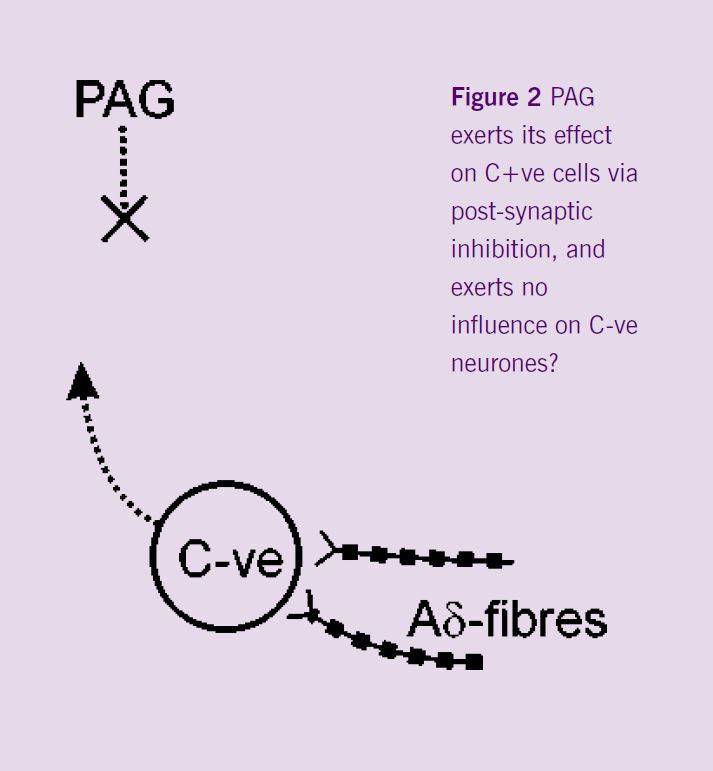
Future experiments will investigate further the effects of PAG activation on C-ve neurones to elucidate precisely the mechanisms involved.
Many thanks to my supervisor, Bridget Lumb, for her assistance with the preparation of this manuscript, and to Dan Simpson, who helped to establish the heating model.