
Physiology News Magazine
Differential screening of a subtractive cDNA library reveals that maternal undernutrition affects fetal heart gene expression
Maternal undernutrition during the first half of gestation induces differential transcription of genes in ovine fetal left ventricular heart. Several of the differentially expressed genes have been associated with hypertrophied adult heart tissue, while the remaining genes have been reported to inhibit hypertrophic growth in adults
Features
Differential screening of a subtractive cDNA library reveals that maternal undernutrition affects fetal heart gene expression
Maternal undernutrition during the first half of gestation induces differential transcription of genes in ovine fetal left ventricular heart. Several of the differentially expressed genes have been associated with hypertrophied adult heart tissue, while the remaining genes have been reported to inhibit hypertrophic growth in adults
Features
Hyungchul Han & Thomas R Hansen
Department of Animal Science, University of Wyoming, Laramie, WY, USA
https://doi.org/10.36866/pn.58.28

Offspring from under-nourished mothers have a predisposition to obesity, diabetes, and cardiovascular disease in adult life. Low weight or thinness at birth in human neonates is associated with increased risk of cardiovascular and metabolic disorders in later life (Barker, 1994). The process whereby the fetus compensates for a maternal insult (undernutrition, stress, etc.) at a sensitive or critical period of fetal development, with consequential long-term effects, has been termed fetal programming. This phenomenon is likely to reflect the benefits of developmental flexibility by the fetus, allowing for short-term survival. However, such adaptations that are beneficial for shortterm fetal survival may be detrimental to health in later life.
A global 50% nutrient restriction during the first half of gestation in sheep resulted in lower fetal weight while both the left and right ventricles of the fetal heart showed compensatory growth by day 78 of gestation (Vonnahme et al. 2003). In order to follow up this observation, we generated a subtractive cDNA library that was enriched for fetal left ventricular heart cDNAs from nutrientrestricted ewes. Screening revealed differential transcription of 11 genes (caveolin, stathmin, cyclin G-1, α-actin, titin, cardiac ankyrin repeat protein, cardiac-specific RNA-helicase activated by MEF2C, endothelial and smooth muscle derived neuropilin, prostatic binding protein, NADH dehydrogenase subunit 2, and an unknown gene) in fetal left ventricle from the nutrient restricted ewes when compared to control fed ewes (Han et al. 2004). The up-regulation of these genes during fetal development may induce hypertrophic growth in the fetal heart as a consequence of maternal undernutrition (Fig. 1).
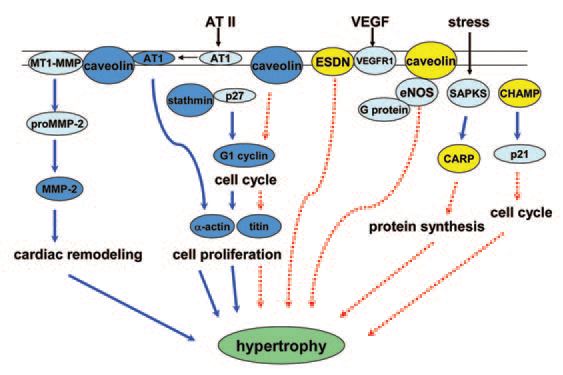
Many of these up-regulated genes have been shown by others to be involved with cardiac hypertrophy in adults. Notably, the remaining genes have actually been reported to inhibit hypertrophic growth in adults. For example, cyclin G1 is up-regulated in nutrient-restricted fetal heart. Cyclin G1 facilitates entry into the cell cycle, thereby increasing cell numbers. In adult heart, cyclin G1 initiates protein synthesis to cause cardiac hypertrophy, rather than DNA synthesis through entry into the cell cycle (Nozato et al. 2000). Cyclin G1 also is up-regulated in growing heart that is still capable of continuing the cell cycle during midgestation.
After birth, heart tissues enter into a stage in which cell numbers do not increase, but size of cells can increase. Thus, genes responsible for hypertrophy may induce hypertrophic growth (increase in cell size) rather than hyperplasia (increase in cell number) in adult heart. Numbers of fetal heart cells increase significantly at mid-gestation. So in the present studies it was not clear if the increase in size of left ventricle was caused by hypertrophic or hyperplastic responses. Interestingly, right ventricular systolic pressure load caused both hyperplastic and hypertrophic growth of right ventricle in near term fetal sheep (Barbera et al. 2000). However, most fetal cardiac tissues near term have already terminated cell division and have entered into a binuclear stage that is similar to adult heart. Future studies of cell size and number would help in interpreting function of fetal left ventricular gene expression in response to maternal undernutrition.
Cardiomyopathy is an important cause of death in the United States and claims more than 27,000 lives annually. Maternal undernutrition during the first half of gestation caused compensatory growth of the left ventricular heart by day 78 of gestation when compared to controls. Whether the changes in gene expression discussed in Fig. 1 are a cardio-protective response in the face of limited nutrient supply, a response to increased systemic vascular resistance and myocyte stretch, or a response to an altered endocrine milieu remains the focus of future investigation. More specifically, the encoded proteins need to be studied to determine if they function as a cause or a consequence of altered left ventricular heart growth and if they represent markers for cardiovascular disease.
Acknowledgements
Research was supported, in part, by NIH BRIN 1P20RR16474. We thank our collaborators on this project: Stephen P Ford and Kathleen J Austin (University of Wyoming) and Peter Nathanielzs and Mark J Nijland (University of Texas Health Sciences Center, San Antonio; NICHD HD21350).
References
Barbera A, Giraud G D, Reller M D, Maylie J, Morton M J & Thornburg K L (2000). Right ventricular systolic pressure load alters myocyte maturation in fetal sheep. Am J Physiol Regul Integr Comp Physiol 279, R1157-1164.
Barker D J (1994). Mothers babies and disease in later life. London, BMJ Publishing group.
Han H C, Austin K J, Nathanielsz P W, Ford S P, Nijland M J & Hansen T R (2004). Maternal nutrient restriction alters gene expression in the ovine fetal heart. J Physiol 558, 111-121.
Nozato T, Ito H, Tamamori M, Adachi S, Abe S, Marumo F, et al. (2000). G1 cyclins are involved in the mechanism of cardiac myocyte hypertrophy induced by angiotensin II. Jpn Circ J 64, 595-601.
Vonnahme K A, Hess B W, Hansen T R, Mccormick R J, Rule D C, Moss G E, et al. (2003). Maternal undernutrition from early- to midgestation leads to growth retardation, cardiac ventricular hypertrophy, and increased liver weight in the fetal sheep. Biol Reprod 69, 133140.
