Physiology News Magazine
Diminishing returns
The rise of the rodent and the importance of larger animal species in biomedical research
Features
Diminishing returns
The rise of the rodent and the importance of larger animal species in biomedical research
Features
https://doi.org/10.36866/pn.124.18
Professor Lucy F Donaldson, University of Nottingham, UK; Co-chair of The Society’s In Vivo Task Force
Professor Ramiro Alberio, University of Nottingham, UK
Dr Jennifer Batson, Exonate Ltd, Cambridge, UK
Dr Frances Henson, University of Cambridge, UK
Professor Roger Lemon, University College London Institute of Neurology, UK
Dr Anna Mitchell, University of Oxford, UK
Dr Jo Murrell, University of Bristol, UK
Dr Domingo Tortonese, University of Bristol, UK
The Physiological Society was founded on 31 March 1876, largely in response to a Royal Commission and legislation on the use of animals in physiological research, which was driven by anti-vivisectionist protests and resulted in the Cruelty to Animals Act of 1876 (The Physiological Society, 2021). At that time, experimental physiology was a rapidly growing discipline following the appointments of new Chairs in Physiology at University College London, and the University of Cambridge. The regulation and legislation surrounding the use of animals in research has, since then, been a constant theme in the work of The Society.
Animals in research and The Physiological Society
In 2018, a Physiological Society meeting at the University of Exeter focused on Experimental Models in Physiology, in which our many participants presented and learned about different experimental models ranging from Antarctic fish to mathematical modelling in physiology (Menzies, 2018). Animal models, particularly but not exclusively mammalian models, have been fundamental to physiology for decades. This landscape is changing rapidly at the moment with a greater emphasis placed on the 3Rs than ever before (the principles of Reduction in animal numbers used in research, the Replacement of animals by other non-animal-based approaches, and the Refinement of existing approaches to minimise pain, distress and lasting harm to animals used in research) and greater importance of the use of species that are most amenable to genetic modification and rapid breeding to produce novel models, principally mice. Physiologists are now successfully using many different methods, models and animals to study function, as well as using non-animal-based methods in line with the 3Rs. Mammalian models remain necessarily important, however, due to the similarities in fundamental physiology between different animal species, and therefore the relevance to human physiology.
The use of animals in physiological and (bio)medical research
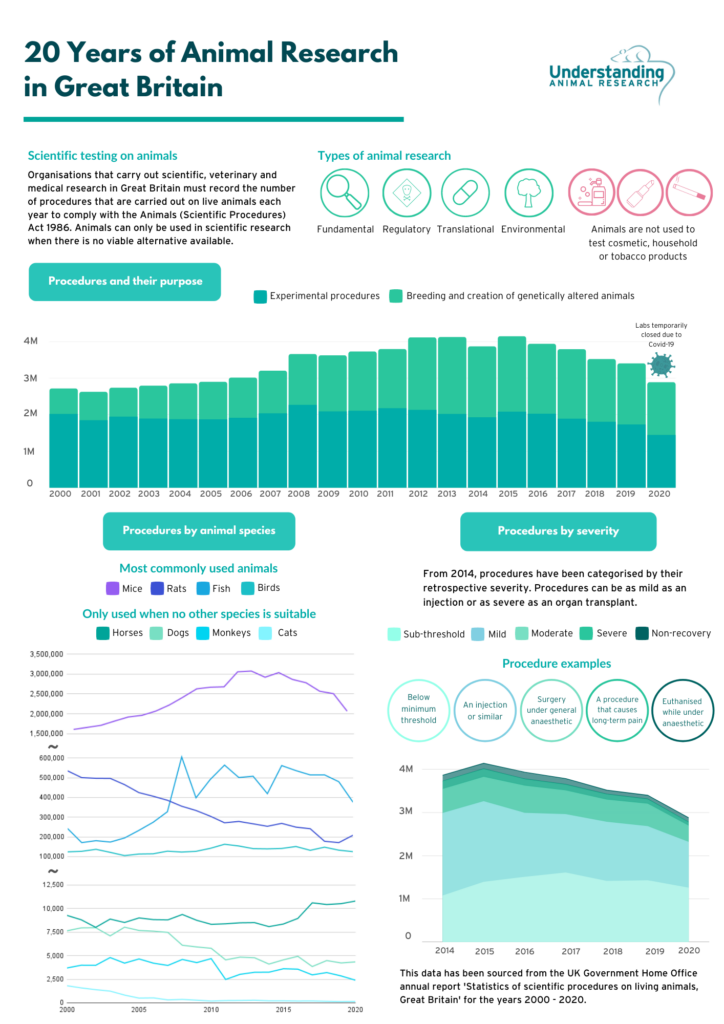
Although there has been legislation protecting animals in research since 1876, the Animals in Scientific Procedures Act (ASPA) 1986, revised and updated to conform with EU Directive 2010/63/EU in 2012, is the core legislation covering the use of animals in science in the UK today. ASPA regulates procedures carried out on protected animals (any living vertebrate, other than man, and any living cephalopod, including embryonic and foetal mammals, birds and reptiles in the last third of gestation or incubation period, larval forms of fish and amphibians that are capable of independent feeding, and cephalopods from the point of hatching). In the most recent UK statistics released by The Home Office (2019), 97% of the 3.4 million procedures on animals involved only three categories of animals: rodents (mice and rats), birds and fish (Understanding Animal Research, 2021).
By far the greatest number of procedures involved mice (2.51 million of the 3.4 million, 74%). Procedures using large species (sheep, rabbits, guinea pigs) and species with special protections (dogs, cat, horses, and primates, which can only be authorised for use under specific circumstances and with oversight from the Home Office) comprised less than 3% of the total number recorded (Fig.1).
This balance in the use of different species was reflected in conversation at the Experimental Models meeting in 2018, where comment was made on the shift to the predominance of mice in biomedical research. This was felt to be a result of both the cost to funders for purchase and housing, as well as ease of genetic modification, making mice the preferred species for most researchers.
But surely, if we use animals in research with the aim of achieving progress in both understanding and in translation of findings to humans and animals, we should always endeavour to use the most appropriate species and experimental model and try to resist pressure to reduce cost as a priority, or to always rely on the outcomes of a genetic manipulation as the strongest evidence for a physiological role.
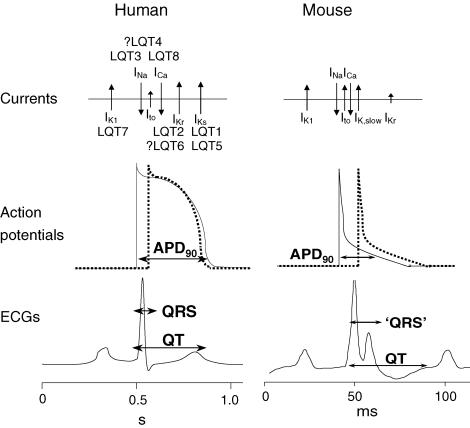
There are notable examples of significant differences in mammalian physiology that make some animals unsuitable for some areas of study. The normal mouse heart rate ranges from 500 to 700 beats per minute (bpm) in contrast to the human rate of 60–100 bpm, with cardiac action potentials in the mouse being 10% of the length of the human, and lacking the calcium plateau that is present in the human. This means that mouse heart and cardiac myocytes are unsuitable for the study of cardiac rhythm or arrythmias. As the cardiac action potential in the rabbit is similar to the human, the rabbit heart is more frequently used in the study of cardiac ion channels, and for example, the effect of drugs on these channels as part of safety testing (Ellerman et al., 2021; Fig.2). This is one example of where a larger animal species is necessary to provide findings directly translatable to humans. There still are strong justifiable needs for the use of larger animals such as pigs, rabbits, sheep, dogs, cats and non-human primates across wider areas of physiological research.
Animal research for animal health and welfare
The need to do research for animals, on animals, for the purposes of improved animal welfare and increased understanding is often overlooked, although this is covered by ASPA. This is not limited to pet and companion animal species of course, but also extends to species used in both agriculture and sport. Endocrine physiology and reproductive seasonality has huge impact on both agricultural breeding and farm animal welfare, and on, maybe surprisingly to PN readers, such issues as jet lag in racehorses.
Both sheep and horses are seasonal breeders displaying strong circannual cycles in their physiology – that is they come into breeding season once per year. Sheep and horses reproduce at opposite times of the year and so investigations on the mechanisms regulating the seasonal physiology in both species can unravel the similarities and differences in their physiology that underlie the successful adaptation to the same changes in the environment. Understanding the control of fertility in sheep and horses can have positive effects on both the animals and on farming, for example, farmers can better understand how to induce sheep fertility at a different time of the year, to delay lambing until the worst of the late winter/early spring weather is over. This can improve the welfare of both sheep and lambs particularly in areas where sheep are not housed indoors during lambing.
Some investigations are possible in these large animals that would certainly not be feasible in rodents let alone zebrafish or fruit flies. Circannual cycles can be readily studied in animals that display endogenous rhythms of hormonal output that are synchronised by external environmental cues. Withdrawal of serial blood samples for the measurements of hypothalamic, pituitary and/or gonadal hormones (Gómez-Brunet et al., 2008) causes less detrimental effect on larger animal well-being than in rodents due to their greater blood volume and therefore the much smaller impact of removing a series of samples from an individual animal and is very difficult in zebrafish due to tiny blood volumes. The feasibility of performing neurosurgery in sheep with a large brain without complications (Lincoln and Tortonese, 1995) or conducting athletic performance tests in horses (Tortonese et al., 2011; Tortonese and Short, 2012) is similar to that in humans, and is much greater in these species than in rodents. These sorts of studies can be used to test hypotheses directly in the species in which the information is to be applied and can produce highly translational and repeatable information.
This argument for the use of larger animals in research also extends to investigation of disease mechanisms and treatments in animals as well as humans, in addition to being important for physiological understanding, enhanced animal welfare and sustainable agricultural production.
Naturally occurring osteoarthritis (OA) is very common in dogs and causes severe pain and disability. However, very little is known about underlying pain mechanisms in dogs suffering from OA, for example, whether the mechanisms of the disease in dogs are similar or different to those in humans. Since so little is understood about this, the development of treatments for OA in dogs is limited. Much of the current osteoarthritis research is conducted using induced models in rats, mice and rabbits, but these may not relate well to spontaneous OA in dogs and indeed humans. Study of mechanisms of OA pain in dogs would not only be of tremendous benefit to the welfare of dogs suffering from this condition, it could also establish whether spontaneous OA in dogs is a valid translational model of OA in humans. This could result in the use of dogs as intermediaries between studies in man and laboratory rodents and would be particularly useful when testing new analgesics or disease-modifying treatments. This could both help overcome the well- known limitations of using induced models of OA in laboratory rodents, increase the development of potential treatments for the dogs themselves (Chakrabarti et al., 2020).
Even larger animals, particularly sheep but also goats and horses, are also used in translational orthopaedic and orthopaedic pain research. Sheep provide opportunities to study individual behaviours in their natural environment rather than in the laboratory, and to contribute sufficient experimental tissue to permit multiple investigations in a single animal, so using fewer animals in the study compared with mice, and consistent with the aims of the 3Rs. Large animal joints are of a similar size, anatomy and physiology to the joint in human, as is the weight borne on the joints. Larger animals are thus important in the development of tissue engineering solutions to treat joint defects, for example, the implantation of biomaterials into cartilage defects and the administration of the quantities of therapeutic cells that could be used clinically. A recent systematic review of such studies in large animals supported the use of these models as effective translational models, particularly for such joint regeneration studies (Veronesi et al., 2020).
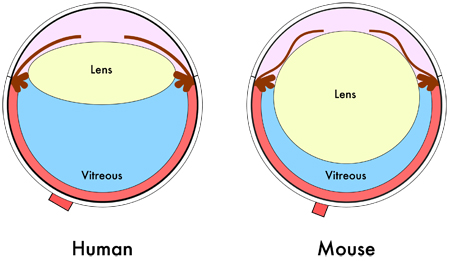
shows the relative smaller vitreous volume in the mouse eye due to its larger lens. [©
2011 JoVE. Skeie et al., 2011. doi.org/10.3791/2795].
Where size really makes a difference
One example of where size can really matter is in the selection of appropriate models for ophthalmology, especially for drug discovery but also for study of physiological responses to retinal damage. Rodent models of eye disease are used and drugs – such as the highly effective wet age-related macular degeneration (wAMD) treatment Lucentis – have been tested in these models and successfully translated into the clinic, these models do not however completely recapitulate the causative processes of human eye conditions that result in blindness, such as wAMD and diabetic macular oedema (DME).
Rodent eyes are obviously much smaller than human eyes, they have thinner cornea and sclera and much lower aqueous and vitreous volumes, plus a large lens occupying a relatively large amount of the intraocular space (Fig.3). Lower vitreous volume hinders the ability to sample vitreous humour to check for example for drug location and/or concentration. Despite these limitations, the use of rodent models of retinal disease in showing new drug efficacy is predictive of translation into humans. Study of animals with larger eyes that more closely mimic human ocular physiology is however essential to ensure that sufficient drug reaches the appropriate target, for example the retina, before progressing to clinical trials, which is necessary for pharmacokinetic study.
In line with the 3Rs, replacement models, such as pig eyes ex vivo, can be used to screen for drug penetration through the wall of the eye, for example if developing eye drops for the treatment of retinal disease. Results from such ex vivo models closely correlate with drug penetration into eyes in vivo and are being used to screen novel drugs for appropriate penetration through the eye, before experiments in vivo. Pharmacokinetic studies are therefore usually done after such ex vivo screening in larger eyes, such as rabbit, in vivo. Rabbit eyes, like rodents, do exhibit significant differences to human eyes, for example rabbits have Harderian glands (found in animals with a nictitating membrane), no macula and differences in retinal architecture, and these differences in structure present some problems for translation. Current best, and required, practice is therefore to confirm the efficacy seen in rodent models and the pharmacokinetic data in rabbits, in non-human primates, prior to phase 1 clinical trial. The non-human primate (NHP) eye has the closest anatomy and retinal vascular system to the human eye and is the only large eye model with a macula, which is the region of the eye affected in wAMD and DME. Models of retinal damage in non-human primates have high predictive translational value. Non-human primate studies obviously require very careful justification, and in-depth, ethical consideration by both NC3Rs and the Animals in Science committee, and the use of highly specialised facilities with highly trained personnel. As a result, such studies are reserved only for final drug molecule efficacy testing prior to clinical trials in ophthalmology.
Genetic modification – not only knockout mice
It has been argued that, because mice can be easily genetically modified, these are the most useful experimental species for this reason alone. There are many researchers for whom the gold standard for demonstration of the importance of a specific gene product in a physiological process is the effect of the complete absence, “knock-out” of that product. The problems of compensation, embryonic lethality and redundancy have been overcome in many cases by the advances in technology allowing selective inducible, tissue and cell-specific knockouts to become more available. There is still a problem of the time and cost to produce the necessary lines when two, three or more genetic alterations are required in a single animal, but this change in approach has led to some very elegant and informative, albeit expensive and involved, experiments, and the application of CRISPR/Cas9 gene-editing technology will continue to revolutionise these approaches.
References
Alberio R and Wolf E (2021). 25th anniversary of clon- ing by somatic-cell nuclear transfer: Nuclear transfer and the development of genetically modified/gene ed- ited livestock. Reproduction 162(1), F59-F68. https://doi.org/10.1530/REP-21-0078
Brekke TD et al., (2018). Inbred or outbred? Genetic diversity in laboratory rodent colonies. G3 8(2), 679- 686. https://doi.org/10.1534/g3.117.300495
Chakrabarti A et al., (2020). Peripheral mechanisms of arthritic pain: A proposal to leverage large animals for in vitro studies. Neurobiology of Pain 8,100051. https://doi.org/10.1016/j.ynpai.2020.100051
Ellerman et al., (2021). Role of the rabbit whole-heart model for electrophysiologic safety pharmacology of non-cardiovascular drugs. Europace 23(6), 828-836. https://doi.org/10.1093/europace/euaa288
Gómez-Brunet A et al., (2008). Endogenous circannual cycles of ovarian activity and changes in prolactin and melatonin secretion in wild and domestic female sheep maintained under a long day photoperiod. Biology of Reproduction 78, 552-562. https://doi.org/10.1095/biolreprod.107.064394
Lincoln GA and Tortonese DJ (1995). Does melato- nin act on dopaminergic pathways in the mediobasal hypothalamus to mediate effects of photoperiod on prolactin secretion in the ram? Neuroendocrinology 62, 425-433. https://doi.org/10.1159/000127032
Mangiavini L et al., (2017). Analysis of mouse growth plate development. Current Protocols in Mouse Biology 6, 67-130. https://doi. org/10.1002/9780470942390.mo150094
Menchaca A et al., (2016). New insights and cur- rent tools for genetically engineered (GE) sheep and goats. Theriogenology 86(1), 160-9. https://doi. org/10.1016/j.theriogenology.2016.04.028
Menzies R (2018). Experimental Models meeting: giv- ing your research a fresh perspective. Physiology News 112: 20. Available at: physoc.org/magazine-articles/experimental-models-meeting-giving-your-research-a-fresh-perspective/
Mitchell AS et al., (2021). International primate neu- roscience research regulation, public engagement and transparency opportunities. Neuroimage 229, 117700. https://doi.org/10.1016/j.neuroimage.2020.117700
The Physiological Society (2021). Society timeline. Available at: physoc.org/about-us/history-archives/society-timeline/
Sykes M, Sachs DH (2019). Transplanting organs from pigs to humans. Science Immunology 4(41), eaau6298. https://doi.org/10.1126/sciimmunol.aau6298
Tortonese DJ et al., (2011). Experimental jetlag disrupts circadian clock genes but improves performance in racehorses after light-dependent rapid resetting of neuroendocrine systems and the rest–activity cycle. Journal of Neuroendocrinology 23(12), 1263-1272. https://doi.org/10.1111/j.1365-2826.2011.02222.x
Tortonese DJ, Short RV. (2012). Biological rhythms, jetlag and performance in Thoroughbred racehorses. Equine Veterinary Journal 44(4), 377-378. https://doi.org/10.1111/j.2042-3306.2012.00589.x
Understanding Animal Research (2021). Numbers of animals. Available at: understandinganimalresearch.org.uk/animals/numbers-animals/
Veronesi F et al., (2020). Meniscectomy-induced osteoarthritis in the sheep model for the investigation of therapeutic strategies: a systematic review. International Orthopaedics 44, 779–793. https://doi.org/10.1007/s00264-020-04493-1
Yao J et al., (2016). Genome editing revolutionize the creation of genetically modified pigs for modeling human diseases. Human Genetics 135, 1093-105. https://doi.org/10.1007/s00439-016-1710-6