Physiology News Magazine
Do peripheral chemoreceptors in the carotid body serve as sites of glucose sensing?
The peripheral chemoreceptors in the carotid body are well known for their role in oxygen sensing and control of respiration. Recently, cellular evidence has shown that these cells are also responsive to glucose. Our recent work is supportive of carotid body involvement in glucose sensing in humans.
Features
Do peripheral chemoreceptors in the carotid body serve as sites of glucose sensing?
The peripheral chemoreceptors in the carotid body are well known for their role in oxygen sensing and control of respiration. Recently, cellular evidence has shown that these cells are also responsive to glucose. Our recent work is supportive of carotid body involvement in glucose sensing in humans.
Features
Erica A. Wehrwein and Michael J. Joyner
Department of Anesthesiology, Mayo Clinic, Rochester, MN, USA
https://doi.org/10.36866/pn.83.13


The brain lives on oxygen, adequate blood pressure and glucose. If any one of these three essential elements for brain function falls below a critical level, unconsciousness and even death can occur. The carotid arteries are located bilaterally in the neck and deliver oxygenated blood and glucose to the brain. They contain strategically located sensory organs that monitor the blood oxygen level (carotid chemoreceptors) before it enters the brain and also blood pressure (carotid baroreceptors). If the carotid chemoreceptors sense a drop in oxygen level, ventilation is stimulated. If a fall in blood pressure is detected by the baroreceptors, there are adjustments in the heart and blood vessels to raise it. The teleological argument is that the carotid chemo- and baroreceptors serve to ensure that blood is delivered to the brain at an appropriate pressure with enough oxygen for neuronal survival. Do the chemoreceptors in the carotid bodies also function to ensure an adequate supply of glucose to the brain?
The carotid body structure is conserved among vertebrates and has also been studied extensively for its role in oxygen sensing. In fish, there are ‘glomus-like’ cells in the first gill arch that serve as an oxygen sensor between the ‘inhaled’ water and blood flow. However, in the case of fish these cells sense the external environment and are unlikely to respond to hypoxaemia and serve a homeostatic mechanism similar to that described above. In diving birds, there is evidence that the carotid bodies and their sensing of oxygen is related to dive duration (Milsom & Burleson, 2007). Furthermore, in diving ducks the carotid bodies mediate at least part of the dive reflex so that oxygen consumption is temporarily slowed and the brain and heart are protected from hypoxic damage. In humans, the carotid bodies play an important role in oxygen sensing as evidenced by the loss of hypoxic ventilatory response in carotid body-denervated patients (Timmers et al. 2003a,b). The latter two are great examples of the role the carotid bodies serve in an integrative physiological response of the neural, respiratory and vascular systems, and underpin the notion that the carotid bodies are important homeostatic control points for key physiological parameters.
Recently cellular evidence has emerged that Type I glomus cells in the carotid bodies are in fact sensors for glucose in addition to their roles in sensing oxygen and carbon dioxide. There is also animal work in support of this notion. In dogs, carotid body resection drastically impairs the animal’s ability to sense and respond to hypoglycaemia (Koyama et al. 2000). During a hypoglycaemic clamp the glucose infusion rate was higher in denervated dogs, consistent with the idea that endogenous glucose production was blunted. There were also blunted glucagon and cortisol responses to hypoglycaemia (Koyama et al. 2000), suggesting that the carotid bodies are integral to the neuro-hormonal response to hypoglycaemia. In a study in exercising dogs, intact carotid bodies were also required for an appropriate neuroendocrine response to exercise and carotid body-resected dogs did not show the normal exercise-induced increase in glucagon, noradrenaline (norepinephrine) or cortisol (Koyama et al. 2001). Given these compelling findings and the strategic location of the carotid bodies, we sought to address the role of the carotid bodies in glucose sensing in conscious humans.
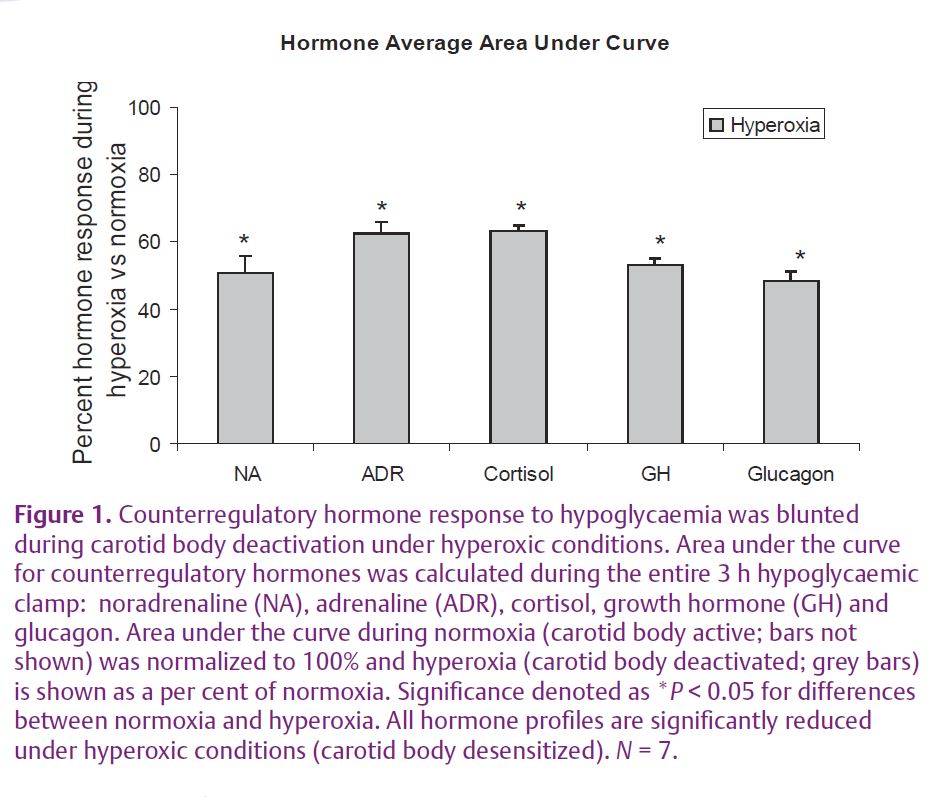
Our recent work in The Journal of Physiology entitled ‘Hyperoxia blunts counterregulation during hypoglycaemia in humans: possible role for the carotid bodies?’ focused on the interactions of oxygen and glucose sensing in the carotid bodies (Wehrwein et al. 2010). Hyperoxia was used to ‘desensitize’ the carotid bodies since exposure to 100% oxygen results in a depression of minute ventilation (Downes & Lambertsen, 1966) and immediately drops chemoreceptor activity in the carotid bodies to zero and initiates a brief apnoea in cats (Lahiri & DeLaney, 1975). When we inactivated the carotid body chemoreceptors, neuro-hormonal counterregulation to hypoglycaemia was blunted (Fig. 1) suggesting: (1) that the carotid bodies play a role in glucose sensing and regulation in humans, and (2) there is an interaction between glucose and oxygen in the carotid bodies. These observations are also consistent with studies from isolated carotid glomus cells showing an additive effect of oxygen and glucose signalling such that the glomus cells respond to hypoglycaemia and that this response can be enhanced with concurrent exposure to hypoxia (Pardal & Lopez-Barneo, 2002).
Before our study there was evidence in healthy humans and in several patient groups for a fundamental link between oxygen and glucose sensing. Although these examples have not been linked to the carotid bodies, it is tempting to speculate that multi-modal sensing in the carotid bodies may be involved. Some examples include: (1) Acute reductions in oxygen saturation in healthy subjects causes glucose intolerance (Oltmanns et al. 2004); (2) In chronic obstructive pulmonary disease (COPD) patients, chronic hypoxia is associated with impaired glucose tolerance while COPD patients with more normoxic blood gases have normal response to oral glucose tolerance testing (Hjalmarsen et al. 1996; Jakobsson & Jorfeldt, 2006); (3) Hypoxic COPD patients acutely placed on supplemental oxygen have an immediate improvement in glucose tolerance and insulin sensitivity during euglycaemic glucose clamps (Jakobsson & Jorfeldt, 2006); (4) Two days of treatment with continuous positive airway pressure improves glucose tolerance and insulin resistance in metabolic syndrome patients with sleep apnoea (Czupryniak et al. 2005; Dorkova et al. 2008); and (5) Diabetic patients receiving insulin and exposed to hyperbaric oxygen for treatment of diabetic ulcers frequently experience unexpected reductions in blood glucose and/ or hypoglycaemia during treatment (Al-Waili et al. 2006). Taken together these observations are consistent with the concept that oxygen and glucose homeostasis are linked, and our recent publication supports the notion that one site of interaction is the carotid bodies. These observations along with our findings also have implications that extend into a variety of diseases in which there is an apparent interaction of oxygen and glucose homeostasis.
In summary, our findings support the cellular, animal and anecdotal clinical evidence that the cells of carotid bodies function as glucose sensors. We have shown an interaction between oxygen and glucose sensing in the carotid bodies with systemic implications. The anatomical location of the carotid bodies makes them ideal for the strategic control of what the brain needs to function in humans and other vertebrates.
References
Al-Waili NS, Butler GJ, Beale J, Abdullah MS, Finkelstein M, Merrow M, Rivera R, Petrillo R, Carrey Z, Lee B & Allen M (2006). Influences of hyperbaric oxygen on blood pressure, heart rate and blood glucose levels in patients with diabetes mellitus and hypertension. Arch Med Res 37, 991–997.
Czupryniak L, Loba J, Pawlowski M, Nowak D & Bialasiewicz P (2005). Treatment with continuous positive airway pressure may affect blood glucose levels in nondiabetic patients with obstructive sleep apnea syndrome. Sleep 28, 601–603.
Dorkova Z, Petrasova D, Molcanyiova A, Popovnakova M & Tkacova R (2008). Effects of CPAP on cardiovascular risk profile in patients with severe obstructive sleep apnea and metabolic syndrome. Chest 134, 686–692.
Downes JJ & Lambertsen CJ (1966). Dynamic characteristics of ventilatory depression in man on abrupt administration of O. J Appl Physiol 21, 447–453.
Hjalmarsen A, Aasebo U, Birkeland K, Sager G & Jorde R (1996). Impaired glucose tolerance in patients with chronic hypoxic pulmonary disease. Diabetes Metab 22, 37–42.
Jakobsson P & Jorfeldt L (2006). Oxygen supplementation increases glucose tolerance during euglycaemic hyperinsulinaemic glucose clamp procedure in patients with severe COPD and chronic hypoxaemia. Clin Physiol Funct Imaging 26, 271–274.
Koyama Y, Coker RH, Denny JC, Lacy DB, Jabbour K, Williams PE & Wasserman DH (2001). Role of carotid bodies in control of the neuroendocrine response to exercise. Am J Physiol Endocrinol Metab 281, E742–E748.
Koyama Y, Coker RH, Stone EE, Lacy DB, Jabbour K, Williams PE & Wasserman DH (2000). Evidence that carotid bodies play an important role in glucoregulation in vivo. Diabetes 49, 1434–1442.
Lahiri S & DeLaney RG (1975). Relationship between carotid chemoreceptor activity and ventilation in the cat. Respir Physiol 24, 267–286.
Milsom WK & Burleson ML (2007). Peripheral arterial chemoreceptors and the evolution of the carotid body. Respir Physiol Neurobiol 157, 4–11.
Oltmanns KM, Gehring H, Rudolf S, Schultes B, Rook S, Schweiger U, Born J, Fehm HL & Peters A (2004). Hypoxia causes glucose intolerance in humans. Am J Respir Crit Care Med 169, 1231–1237.
Pardal R & Lopez-Barneo J (2002). Low glucose-sensing cells in the carotid body. Nat Neurosci 5, 197–198.
Timmers HJ, Karemaker JM, Wieling W, Marres HA, Folgering HT & Lenders JW (2003a). Baroreflex and chemoreflex function after bilateral carotid body tumor resection. J Hypertens 21, 591–599.
Timmers HJ, Wieling W, Karemaker JM & Lenders JW (2003b). Denervation of carotid baro- and chemoreceptors in humans. J Physiol 553, 3–11.
Wehrwein EA, Basu R, Basu A, Curry TB, Rizza RA & Joyner MJ (2010). Hyperoxia blunts counterregulation during hypoglycaemia in humans: possible role for the carotid bodies? J Physiol 588, 4593–4601. http://jp.physoc.org/content/588/22/4593.long