
Physiology News Magazine
Does muscle pain increase muscle stiffness?
Animal studies have shown that muscle pain can reflexly excite gamma motor neurones and thus increase muscle spindle stretch sensitivity and discharge rate. According to the popular, but clinically unproven, hypothesis this reflex loop may perpetuate into a sustained 'vicious cycle' - reciprocally aggravating muscle tone and pain. Yet a lack of clear experimental evidence – and recently obtained new data in humans that contradicts this notion – has cast doubt on whether this model can be used to explain physiological mechanisms and translate into the development of treatments
Features
Does muscle pain increase muscle stiffness?
Animal studies have shown that muscle pain can reflexly excite gamma motor neurones and thus increase muscle spindle stretch sensitivity and discharge rate. According to the popular, but clinically unproven, hypothesis this reflex loop may perpetuate into a sustained 'vicious cycle' - reciprocally aggravating muscle tone and pain. Yet a lack of clear experimental evidence – and recently obtained new data in humans that contradicts this notion – has cast doubt on whether this model can be used to explain physiological mechanisms and translate into the development of treatments
Features
Ingvars Birznieks (1), Alexander R Burton (1,2) and Vaughan G Macefield (1,2)
1: Prince of Wales Medical Research Institute, Sydney, Australia
2: School of Medicine, University of Western Sydney, Sydney, Australia
https://doi.org/10.36866/pn.73.21

Chronic musculoskeletal pain syndromes (CMPS) are a common group of, usually, activity and work-related myalgias. Unfortunately, the efficiency of treatments available today is very limited, partly because the physiological mechanisms underlying the clinical conditions are not well understood. One of the hypotheses – developed by Peter Sojka and the late Håkan Johansson in Umeå, Sweden – suggests that chronic muscle pain may develop as a result of a ‘vicious cycle’ reflex initiated by nociceptive input itself (Johansson & Sojka, 1991). This hypothesis is based on an elegant set of experiments in the anaesthetised animals demonstrating that nociceptive afferents excite γ-motor neurones and thereby increase stretch sensitivity and discharge rate of the muscle spindles. The resultant excitation of the homonymous α-motoneurone pool may lead to increased muscle tone, contraction-induced ischemia and accumulation of metabolites (Johansson & Sojka, 1991). If the production of metabolites is reasonably high to excite nociceptors, a process sustaining a ‘vicious cycle’ might be initiated, resulting in a chronic muscle pain.
In contrast to animal experiments, to date clear experimental evidence in humans is lacking. Variable results have been obtained when attempting to demonstrate these effects in human subjects by looking at EMG activity, stretch reflexes and proprioceptive function; nevertheless, they all agree that the ‘vicious cycle’ mechanism is very unlikely.
Due to methodological limitations the conclusions in regard to modulation of muscle spindle activity in humans so far have been based on indirect experimental evidence. However, our recent study has overcome those constraints by using microneurography to record discharge activity of single human muscle spindle afferents during experimentally-induced pain in muscle and skin (Fig. 1) (Birznieks et al. 2008).
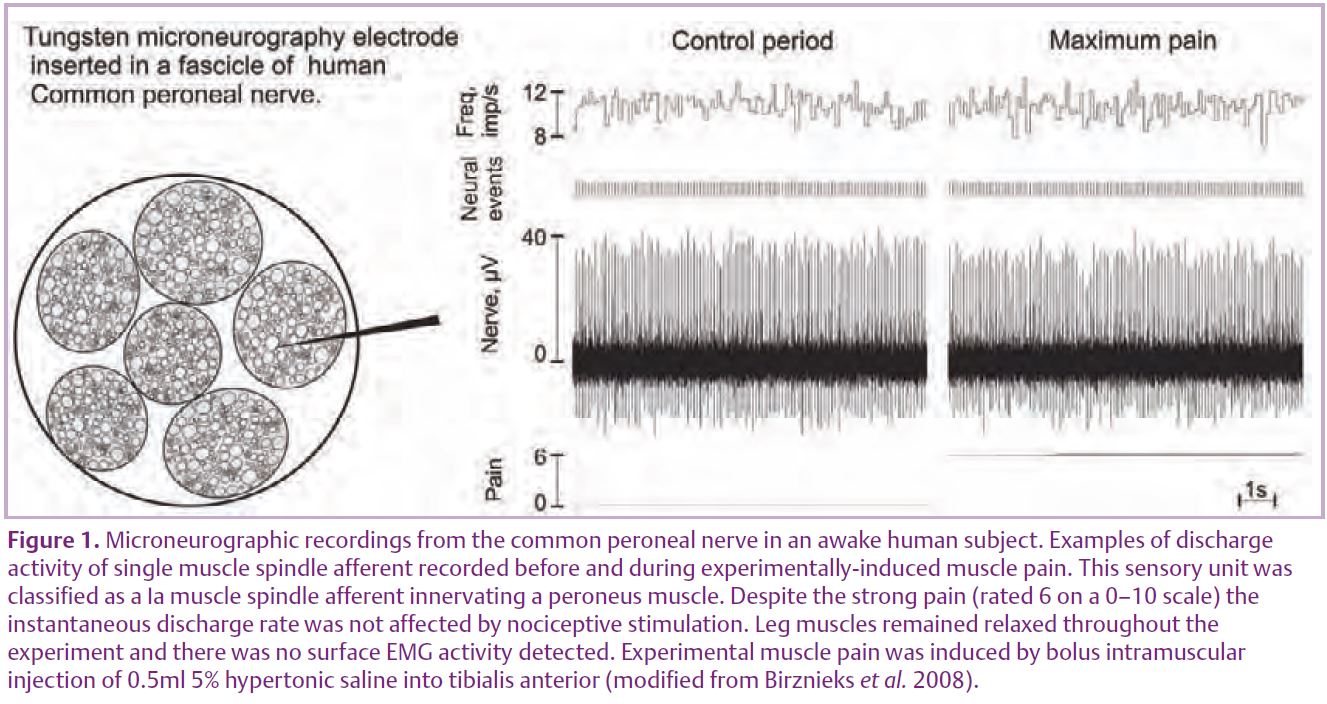
Unitary recordings were made from 14 primary and six secondary muscle spindle afferents, located in the ankle or toe extensors or the peronei muscles, via microelectrodes inserted into the common peroneal nerve. During muscle pain, induced by intramuscular injection of 0.5 ml of 5% hypertonic saline, no afferents increased their discharge activity in response to static stretch, moreover the overall net discharge rate decreased during muscle pain by 6.1%. During skin pain, induced by a subcutaneous injection (0.2 ml), only small changes in afferent activity were observed and the overall net discharge rate remained essentially the same.
To support the ‘vicious cycle ’ hypothesis, nociceptive excitation of fusimotor drive must be substantial. Experiments on anaesthetised cats, conducted in a comparable experimental setup to our study, demonstrated a substantial reflexogenic increase in mean discharge rate by ~80% in afferents innervating homonymous as well heteronymous muscles (Thunberg et al. 2002). This was not the case in our human microneurography experiments, firmly contradicting animal data and thus questioning the clinical relevance of the ‘vicious cycle’ hypothesis (Birznieks et al. 2008).
An intriguing question to address in the future is what physiological mechanisms are responsible for such discrepancies between species and experimental approaches reported over the years? In the discussion of our article (Birznieks et al. 2008) we touch upon several possibilities and consider an adaptive peripheral reflex control of the fusimotor system that reflects a spectrum of function and adaptation depending on the context.
An important aspect of human experiments is voluntary control, which hypothetically might counteract nociceptive activation of α and γ-motor neurones. Apart from the fusimotor system, another less explored pathway for pain to modulate muscle spindle activity might be via the sympathetic nervous system. In our paper we review the studies advocating this possibility, but at the same time we have to point out contradictory and inconsistent findings that prevent us from drawing any definite conclusions. All previous studies have been designed to focus on either fusimotor or sympathetic influences on muscle spindles separately – a unified model taking into account of both mechanisms would probably provide a better explanation of experimental results.
While the major support to the Johansson/Sojka hypothesis has been provided by the observation of immediate reflexogenic responses, which, as we now know, are not present in humans, there is the possibility that recruitment of ©-motor neurones might require plastic changes in nociceptive circuits that might develop over a longer time. However, prolonged experimental pain in animals involving inflammatory agents seem to inhibit γ-motor neurons, thus actually providing an explanation for the weakness and even the atrophy clinically observed in severe chronic cases of muscle damage.
Finally, what about the clinical evidence – can any convincing support for the ‘vicious cycle’ hypothesis be found? While it is common knowledge that involuntary muscle contractions are seen in patients suffering from pain, something other than the muscle pain itself might be causing and maintaining a spasm as, for example, abdominal rigidity is associated with peritoneal inflammation. Simons & Mense (1998) indicated that usually painful muscle shows no EMG activity and, if it is present, it does not correlate with pain either in the time or intensity domain.
Furthermore, Lund et al. (1991) reviewed a wide range of clinical literature and experimental studies and came to the conclusion that chronic pain tends to inhibit, not facilitate, voluntary and reflex contractile activity of a painful muscle or its agonists. These authors suggest that those effects are beneficial and provide protective adaptation, and are definitely not the cause of pain.
In conclusion, due to the controversy and conflicting results reported over the years, a final chapter on this issue is far from being written yet.
However, now there is new direct experimental evidence indicating that the key mechanism – on which the clinical ‘vicious cycle’ hypothesis is based – is missing; in humans activation of muscle nociceptors does not cause a reflex increase in fusimotor drive. Thus we urge caution in extending animal data to the clinical setting.
References
Birznieks I, Burton AR & Macefield VG (2008). The effects of experimental muscle and skin pain on the static stretch sensitivity of human muscle spindles in relaxed leg muscles. J Physiol 586, 2713–2723.
Johansson H & Sojka P (1991). Pathophysiological mechanisms involved in genesis and spread of muscular tension in occupational muscle pain and in chronic musculoskeletal pain syndromes: a hypothesis. Med Hypotheses 35, 196–203.
Lund JP, Donga R, Widmer CG & Stohler CS (1991). The pain-adaptation model: a discussion of the relationship between chronic musculoskeletal pain and motor activity. Can J Physiol Pharmacol 69, 683–694.
Simons DG & Mense S (1998). Understanding and measurement of muscle tone as related to clinical muscle pain. Pain 75, 1–17.
Thunberg J, Ljubisavljevic M, Djupsjöbacka M & Johansson H (2002). Effects on the fusimotor-muscle spindle system induced by intramuscular injections of hypertonic saline. Exp Brain Res 142, 319–326.
