
Physiology News Magazine
Drosophila – a model for all reasons
This tiny fly is too useful to be the preserve of developmental biology
Features
Drosophila – a model for all reasons
This tiny fly is too useful to be the preserve of developmental biology
Features
Julian Dow
Institute of Molecular, Cell & Systems Biology, University of Glasgow, UK
https://doi.org/10.36866/pn.99.20
For many years, physiology and genetics were on orthogonal trajectories. However, combining the two approaches can provide valuable general insights. Nowhere is this better illustrated than in the fruit fly.

The Krogh principle and comparative physiology
August Krogh (Nobel prize in Medicine, 1920, photo Fig 1) is widely considered to be the founding father of modern comparative physiology, and his eloquent exposition of the comparative approach ‘for such a large number of problems there will be some animal of choice, or a few such animals, on which it can be most conveniently studied’, is now known as the Krogh principle. In the current era, where biomedical relevance is key, it is easy to forget that physiological advances have come from anything other than mouse; however, Krogh’s principle of choosing the best species for the question being studied shines through some of our biggest advances – the squid giant axon, the electric ray electric organ, frog skin and snail neurones have all advanced our understanding of the human condition more than we might think. Indeed, this principle of using a simple model for a general insight goes further back in history; the great natural philosophers of the enlightenment would draw, not just on different species, but different sciences, as they saw fit.
Drosophila as a ‘Krogh’ organism
On the surface, it might seem that Drosophila has very little to commend itself as an ‘ideal’ physiological subject. It is small –tiny, in fact as shown in Fig 2 – and an utterly harmless and undistinguished member of one of the largest Orders of insects. The rapid generation time (1 week) and fecundity of this little fly (one mating can produce several hundred offspring) means that genetics is really powerful, and their small size and the low cost of rearing them means that a modest fly group can keep hundreds of mutant lines – something that is not so easy with transgenic mice! So Drosophila is a genetic model par excellence. As it develops from fertilized egg to larva in just 22 hours, it has become the darling of developmental biologists (and indeed, underpinned a Nobel prize). Although this explains the continued fixation of most of the Drosophila community with development, the organism is really far more versatile! Indeed, the fruit fly is already embedded in our physiological lexicon. The reason is that, with genetic power comes the ability to see mutations, or to perform screens, that provide overwhelming insights with great speed and agility, compared with our bigger models. Below, I give a few examples.

White
Back in 1910, Thomas Hunt Morgan, working in the new field of Genetics at Columbia, spotted the first Drosophila mutant, white. Instead of the rather lovely red eyes characteristic of the species, these eyes were brilliant white. The mutation was a classical Mendelian recessive; and so the floodgates opened on Drosophila genetics. But what does white actually encode? It’s a broad-spectrum organic solute transporter of the ABC family, instantly recognisable today as an orthologue of our own ABC transporters. The precursors of the bright red eye pigments are stored and metabolized in the kidney tubules of the rapidly growing larva, then released into the pupal blood space to be taken up by the maturing compound eye. White plays a role in these transport processes; in mutants, the kidney tubules fail to accumulate pigment precursors (and so are white instead of yellow) – and in turn, the eye never gets any pigment to absorb, and so also looks white. So, long after Morgan’s discovery, we can trace out a physiological odyssey of transporters, tissues and metabolites. Better yet, although there are now hundreds of alleles of white, the direct descendants of the very first fly that Morgan spotted are still available from Drosophila stock centres by post. For $25 – just ask for white1. Talk about walking in the footsteps of giants…
Shaker and voltage-gated ion channels
Another spontaneous Drosophila mutant transformed our understanding of neurobiology ( Bellen HJ, Tong C, & Tsuda H, 2010). In 1944, Catsch described a line of flies bred from a single fly observed to shake its legs under ether anaesthesia (before boring old carbon dioxide anaesthesia, Drosophila labs were quite fragrant –and dangerous – places). The mutant was named Shaker, and subsequent decades of neurophysiological research showed that it was defective in rapidly inactivating (A type) potassium conductance – hence the shaking. One of the great early triumphs of molecular biology had been the sequencing of the first sodium channel, with its highly suggestive structure of four repeats of 6 transmembrane domains. The internal similarity of these four repeats suggested that the gene had evolved by two successive tandem gene duplications of an ancestral channel of just 6 transmembrane domains. So, instead of making a 24-helix tetramer of 4 separate 6-helix proteins, we had evolved a single gene encoding all 24. When Shaker was sequenced, this turned out to be the ‘living fossil’ ancestral condition – it encoded 6 helices that operated as a subunit of a homotetramer. However, it would be quite incorrect to think of Shaker as the primitive condition – flies are no less ‘evolved’ than us! We diverged from flies 400 M years ago, and we are both quite successfully alive today. And in due course, a six transmembrane domain Shaker orthologue was identified in humans. Today, of course, we know of the Sh, Shal, Shab and Shaw families of 6-transmembrane potassium channels, common to flies and humans.
Dunce, cabbage, rutabaga – the molecular basis of learning and memory
These first two stories were about chance discoveries of spontaneous mutants. However, it is relatively easy to mutate thousands of flies with X-rays or chemical mutagens, to screen them for individual mutants with a desired phenotype, then to painstakingly identify the mutated gene – this is known as classical, or forward, genetics. Such screens paved the way for our understanding of learning and memory. Drosophila are not as stupid as one might think, and can be trained to associate particular odours or tastes with a mild electric shock, and this was the basis for a screen that identified the first learning and memory mutants in any species. Flies learn the odour shock pairing, in a T-maze, in relatively few trials, then forget relatively slowly; it is thus possible to screen either for flies that are defective in learning separate from those that learn normally, but forget quickly. Such screens identified dunce, the archetypal memory mutant. Dunce encodes a cyclic AMP phosphodiesterase, firmly pointing the finger at the cAMP signalling pathway as key in associative learning. Other learning mutants – wittily named after vegetables – support this theme: rutabaga encodes an adenylate cyclase, for example. Critically, these findings resonated with those of Nobel laureate Kandel on the simple sea slug Aplysia, confirming a broad phylogenetic base for cAMP in learning and memory.
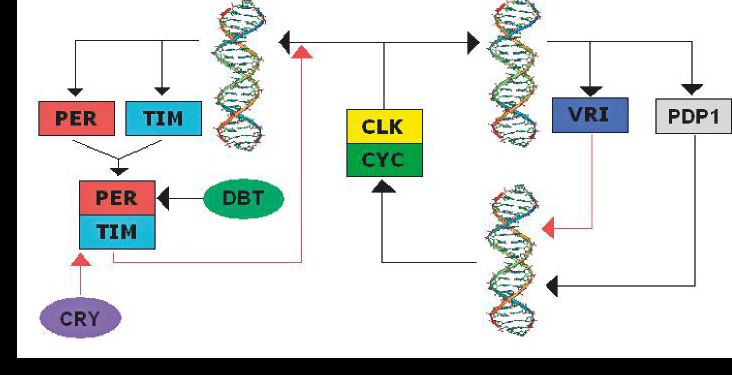
Circadian rhythms and clock genes
Now that the principle of identifying genes based on an informative screen is established, we can have more fun. Drosophila adults tend to emerge from their pupae at a particular time of day, and adults show clear diurnal locomotor patterns. These behaviours persist in constant darkness. So screening mutants for locomotor behaviour that fails to show circadian rhythmicity in darkness could identify
genes that are important in intrinsic clocks. That describes nicely the discovery of the archetypal clock gene, period, and its partners in crime, such as clock and timeless, see Fig 3. Such genes have been found from plants to flies to humans, so the principle established in a simple model is of general application.
Reverse genetics and the Drosophila ‘toolbox’ for integrative physiology
The forward genetics method is powerful, but slow; until the advent of next-generation sequencing, it could take a decade to identify the DNA change associated with a mutation. Reverse genetics is the opposite; a gene is intentionally mutated in the hope that the resulting phenotype will tell us what the gene does. This is vital for modern biology, because surprisingly, we have no idea of the function of about a fifth of our 20 000 or so genes. Now, creating mutations in humans is not a good path to tread; so reverse genetics of novel gene orthologues in genetic model organisms is a hugely important process. Indeed, this is precisely why mouse is now such an important model.

In Drosophila, much use has been made of transposons, autonomous bits of DNA that insert in our genomes randomly. Transposons encode transposase, the enzyme that catalyses their excision and reinsertion with relatively high efficiency. Drosophila research makes heavy use of one such transposon, the P-element. The transposase can be replaced with a genetic payload of choice; and when co-injected into fly embryos with a separate source of transposase, the P-element can insert into the genome with high efficiency. As well as a simple method for high-efficiency transgenesis, P-element insertions near to a gene of interest can be identified by PCR, providing a rapid means to mutate a specific gene.
One day in the early 1990s, my late colleague Kim Kaiser explained to me a method that allowed expression of any transgene in any tissue or cell population of the experimenter’s choice, and so convinced me to jump ship to Drosophila. As a comparative physiologist, I had wanted to tinker with genes in just the way that we can pharmacologically intervene with some signalling pathways – but without the clumsiness associated with simply mutating the whole gene. Classical mutations can have multiple effects on multiple tissues (this is called pleiotropy); but the GAL4/UAS binary system allows beautifully precise interventions to be made ( Duffy JB , 2002). Essentially, the yeast transcription factor GAL4 is put under control of a gene with a tissue or cell-specific expression pattern, and so is expressed in the same pattern of cells; such GAL4 lines are typically identified as part of a large-scale P-element screen. GAL4 has no major effects in the fly genome; but when such GAL4 ‘driver’ lines are crossed to flies transgenic for P-elements containing a genetic payload downstream of UAS, the sequence bound by GAL4, something magical happens. In the progeny of the cross, the genetic payload is expressed in only those cells in which GAL4 is being expressed. So all Drosophila labs keep a panel of GAL4 driver fly lines that can direct expression of transgenes of choice, simply by performing a cross, see Fig 4.
Better yet, generating such UAS lines is not a long job; there are off-the-shelf vectors for most purposes, and injection services that will produce transgenic flies from your plasmid in about 3 months, for less than $500. So (for example) a graduate student in my lab could fuse their gene of interest to a GFP gene in a UAS vector and send off the construct to be injected, in about a week, and three months later could be studying the subcellular localization of their protein in specific cells of an otherwise normal organism. By contrast with mouse transgenics, I would not necessarily need to approve such a modest expense! This freedom to perform cell-specific interventions in an otherwise normal organism is what I call the ‘toolbox’ of integrative physiology. Of course, the GAL4/UAS system is only one simple example of what can be achieved, and the book is being rewritten all the time; many Drosophila labs, for example, are currently excited about genome editing with CRISPR/Cas9. No matter the detailed technology, the physiological uses are limited only by one’s imagination.

Harnessing the toolbox for real physiology
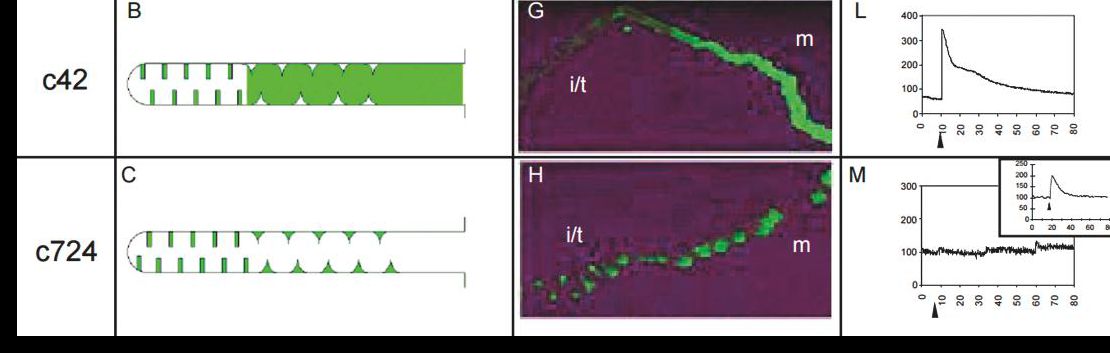
My lab, together with that of Shireen Davies, focusses on integrative physiology and functional genomics of osmoregulation and renal function. Scaling arguments suggest that terrestrial insects live their lives in continual danger of drying up, and so osmoregulation is critical to their success. Paradoxically, then, insect renal tubule cells can secrete their own volume of primary urine every 6 seconds, the fastest rate known for any epithelium (Beyenbach, Skaer, & Dow 2010). So both the transport mechanisms and their tight neuroendocrine control are of great interest, both from the basic science viewpoint, and because disruption of these control mechanisms could provide us with valuable new insecticides. Our GAL4 driver lines demarcate the major cell types and regions in this tiny tissue of 150 cells, and we have used it, for example to make the first animals transgenic for a calcium reporter, allowing us to show that a particular neuropeptide signals through calcium in only one cell type in the tissue, see Fig 5.
Biomedicine
Across 400 My of divergent evolution, it might seem that there would be little in common between Drosophila and human renal function. Indeed, you won’t find renin or angiotensin in flies. However, the two tissues perform analogous tasks, with orthologous genes, and so there can be some surprises. For example, simply by feeding flies oxalate, we have been able to model in flies the most common form of kidney stones, with the advantage that they are visible in situ, as shown in Fig 6, and not hidden in an opaque kidney capsule (Dow & Romero MF, 2010). This unique advantage opens the door to a screen for agents that block stone formation.
However, it is important to appreciate that Drosophila is not important just because we can model human function or disease; Drosophila is a highly successful organism, and understanding how it works is important in the bigger scheme of things. Although biomedical imperatives have distorted the picture, human physiology is but a special case of physiology. Great minds have appreciated this: indeed, the famous codebreaker Alan Turing studied pattern formation in Drosophila, and proposed in 1952 that diffusible morphogens provided a self-organising system. And our system, the Malpighian tubule, is named after Marcello Malpighi, in the 17th century the physician to the pope and the father of microscopic morphology. He studied insect and human kidneys with equal enthusiasm; and several structures, discovered by him, bear his name today.
Acknowledgements
This work is funded by the NIH, BBSRC, and European Union Horizon 2020.
References
Rosay P, et al. (1997). Cell-type specific calcium signalling in a Drosophila epithelium. J. Cell Sci. 110 (Pt 15):1683-1692
Bellen HJ, Tong C, & Tsuda H (2010). 100 years of Drosophila research and its impact on vertebrate neuroscience: a history lesson for the future. Nat Rev Neurosci 11(7):514-522
Duffy JB (2002). GAL4 system in Drosophila: a fly geneticist’s Swiss army knife. Genesis 34(1-2):1-15
Beyenbach KW, Skaer H, & Dow JA (2010). The developmental, molecular, and transport biology of Malpighian tubules. Annual review of entomology 55:351-374
Dow JAT & Romero MF (2010 ). Drosophila provides rapid modeling of renal development, function, and disease. Am J Physiol Renal Physiol 299(6):F1237-1244
