Physiology News Magazine
Elastin and hypertension: is there a link?
Recent work has highlighted the association between a defect in elastin synthesis, large artery abnormalities and hypertension development. Studies in spontaneously hypertensive rats also point in the same direction and suggest a link between a defect in elastin organization and resistance artery remodelling
Features
Elastin and hypertension: is there a link?
Recent work has highlighted the association between a defect in elastin synthesis, large artery abnormalities and hypertension development. Studies in spontaneously hypertensive rats also point in the same direction and suggest a link between a defect in elastin organization and resistance artery remodelling
Features
Silvia M. Arribas (1), Ana M. Briones (2), José M. González (1)
1: Departamento de Fisiología
2: Departamento de Farmacología y Terapéutica Facultad de Medicina, Universidad Autónoma de Madrid, Spain
https://doi.org/10.36866/pn.56.25
The physiological demands of the arterial system in vertebrates require that arteries store energy during systole and release it during diastole, enabling the system to maintain a continuous blood flow. This function is made possible by vessel elasticity, which depends largely on the presence of elastin in the vessel wall. Elastin is synthesized by smooth muscle cells, and is secreted as the soluble monomer tropoelastin, which is then cross-linked in the tissue space and associated with microfibrillar proteins to form insoluble elastin matrix. In large vessels elastin is organized into concentric rings of elastic lamellae around the arterial lumen. Each elastic lamella alternates with a ring of smooth muscle, forming a lamellar unit that provides the compliance that arteries need to absorb and transmit hemodynamic forces (Parks et al. 1993).
The suggested link between a defect in elastin synthesis during early development and hypertension was proposed some time ago, and is based on the evidence that people with low birth weight tend to have higher blood pressure later in life. One mechanism that might underlie this association is impaired elastin synthesis when growth is retarded during a critical period of blood vessel formation in the fetus, leading to abnormal large artery compliance and finally higher blood pressure (Martin & Greenwald, 1997).
Hypertension is also present in a proportion of patients with two clinical conditions associated with elastin gene defects: supravalvular aortic stenosis (SVAS) and Williams-Beuren syndrome. Often individuals with these diseases are young children who are susceptible to peripheral vascular disease, myocardial infarctions or stroke (Milewicz et al. 2000).
A recent study of mice heterozygous for the elastin gene (eln+/-) – a murine model of SVAS – has provided good evidence for a link between elastin and hypertension (Faury et al. 2003). In this study, Faury and co-workers demonstrate that elastin haploinsufficiency results in abnormalities in large artery mechanical properties which lead to a hypertensive phenotype from birth.
These data suggest that any factor that reduces elastin during a critical window of vessel wall formation and alters large artery compliance could have a modifying effect on the progression of, or susceptibility to, hypertension. As elastin is the dominant extracellular protein in large arteries, the effects of elastin defect on these vessels is to be expected. However, we were very surprised to find that elastin also plays a critical role in the mechanical properties and structure of resistance arteries, where this protein is very scarce. Small arteries play an essential role in blood pressure regulation by contributing to total peripheral resistance. In fact, increased peripheral resistance due to resistance-artery narrowing – termed ‘inward vascular remodelling’ – is a key determinant for the maintenance and progression of hypertension, and possibly its development (Mulvany, 2002).
Our recent data demonstrate a link between altered elastin organization in mesenteric resistance arteries (MRA) from spontaneously hypertensive rats (SHR, a model of human essential hypertension) and vascular remodelling (Briones et al. 2003). Fluorescent confocal microscopy enables examination of relatively thick intact tissues without the need to cut sections. We have taken advantage of this and of the autofluorescent properties of elastin (excitation 488nm, emission 515 nm) to determine the organization and distribution of this protein in intact MRAs from SHR and from the normotensive reference strain, WKY, fixed at physiological pressures. In MRA elastin was restricted to a thin internal elastic lamina, a loose network of elastin fibres in the adventitia (external elastic lamina) and some fibres in the medial layer (Fig. 1). SHR MRAs showed altered internal elastic lamina organization with smaller fenestra when compared to the normotensive strain (Fig. 1).
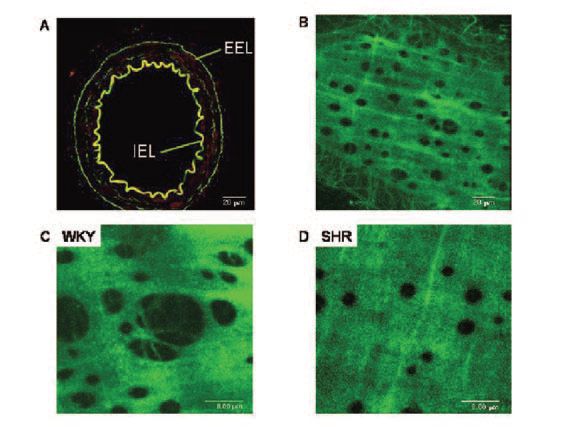
However, elastin content, estimated from fluorescent intensities, was similar between strains. This altered elastin organization was associated with reduced lumen diameter and with increased stiffness in adult SHR, as shown by the leftward shift of the stress-strain relationship and larger β values (Fig. 2).

The importance of elastin organization in resistance arteries is emphasized by the fact that elastin digestion with elastase for 1 h induced a dramatic increase in lumen size and in β value, suggesting that elastin has an unanticipated role in overall small artery dimensions and mechanical properties. More interestingly, elastase abolished the structural and mechanical differences between hypertensive and normotensive vessels, supporting the hypothesis of a link between inward remodelling and a defect in elastin organization (Fig. 2) (Briones et al. 2003).
Our recent data demonstrate that this defect occurs between the 2nd and 4th week of life, when SHR rats are in the pre-hypertensive phase and small artery dimensions are still similar between strains (González et al. 2003). These data suggest that abnormal elastin organization occurs prior to inward remodelling, and might contribute to it.
All these data point to the potential importance of elastin or proteins related to elastic fibre assembly and organization, and the genes that control these, as critical issues in understanding vascular remodelling in essential hypertension.
References
Briones AM, González JM, Somoza B, Giraldo J, Daly CJ, Vila E, González MC, McGrath JC & Arribas SM (2003). Role of elastin in spontaneously hypertensive rat small mesenteric artery remodeling. J Physiol 552, 185-195.
Faury G, Pezet M, Knutsen RH, Boyle WA, Heximer SP, McLean SE, Minkes RK, Blumer KJ, Kovacs A, Kelly DP, Li DY, Starcher B & Mecham RP (2003). Developmental adaptation of the mouse cardiovascular system to elastin haploinsufficiency. J Clin Invest 112, 1419-1428.
González JM, Somoza B, Fernández-Alfonso MS, Gálvez B, Abderrahim F, González MC & Arribas SM (2003). Elastin reorganization and vessels stiffness is an early event in the development of vascular remodeling in resistance arteries from SHR. Hypertension 42, 387.
Martin CN & Greenwald SE (1997). Impaired synthesis of elastin in walls of aorta and large conduit arteries during early development as an initiating event in pathogenesis of systemic hypertension. Lancet 350, 953-955.
Milewicz DM, Urbán Z & Boyd C (2000). Genetic disorders of the elastic fibre system. Matrix Biology 19, 471-480.
Mulvany MJ (2002). Small artery remodeling in hypertension. Curr Hypertens Rep 4, 49-55.
Parks WC, Pierce RA, Lee KA & Mecham RP (1993). Elastin. Advances in Mol Cell Biol 6, 133-182.