
Physiology News Magazine
Erythropoietin controls ventilation in hypoxia
The presence of erythropoietin in blood and brain increases the ventilatory response of mice exposed to oxygen deprivation. Expression of the Epo receptor in the respiratory centre as well as in the carotid bodies suggests that Epo controls the hypoxic ventilatory response at the central (brainstem) and peripheral (carotid body) level
Features
Erythropoietin controls ventilation in hypoxia
The presence of erythropoietin in blood and brain increases the ventilatory response of mice exposed to oxygen deprivation. Expression of the Epo receptor in the respiratory centre as well as in the carotid bodies suggests that Epo controls the hypoxic ventilatory response at the central (brainstem) and peripheral (carotid body) level
Features
Jorge Soliz (1), Joseph Vincent (2), Omolara Ogunshola (1), Max Gassmann (1)
1: Institute of Veterinary Physiology, Vetsuisse Faculty, and Zurich Center for Integrative Human Physiology (ZIHP), University of Zurich, Switzerland
2: Department of Paediatrics, Laval University, Centre de Recherche, Hopital St-Francois d’Assise, Quebec, Canada
https://doi.org/10.36866/pn.63.20

Erythropoietin (Epo) does far more than ‘just’ increasing red blood cell number. Originally discovered as a
‘blood hormone’ and widely applied to anaemic patients, Epo has the potential to become a multipurpose drug. It all began back in 1993, when Ryuzo Sasaki and his Japanese co-workers reported the presence of a functional Epo receptor in rat pheochromocytoma PC12 cells, an established cell line with neuronal characteristics (Sasaki et al. 2001). A few years later Sasaki’s research team, as well as our own, demonstrated that Epo and its receptor are both expressed in the mammalian brain, including human, and that cerebral Epo is expressed in an oxygendependent manner. Considering that the blood-brain-barrier cannot be crossed by Epo, we predicted a local function of Epo in the brain upon binding to its receptor, but which one(s)?
Several elegant experiments performed by various teams on different rodent models of stroke revealed that Epo exerts a protective function upon experimentally-induced brain ischemia. As early as 2002, Hannelore Ehrenreich and her team from Göttingen (Germany) published the first, and so far unique, report on Epo’s neuroprotective function in man. Application of recombinant human Epo (rhEpo) in stroke patients was beneficial when given within eight hours of the ischemic insult (Ehrenreich et al. 2002). Apart from the tendency to reduce the infarct volume, several clinical tests indicated that Epo-treated patients recovered better and faster compared to the saline-treated control patients.
Because Epo has neuroprotective effects, we and others tested the impact of rhEpo on other neuronal tissues such as the spinal cord and the retina (reviewed in Gassmann et al. 2003). As predicted, we observed that the Epo receptor is also expressed in the retina. Surprisingly, an increased level of Epo in the retina was beneficial as it prevented mice from light-induced retinal degeneration (Grimm et al. 2002). Epo’s protective effects are even more widespread, as it has been shown by different teams that Epo exerts beneficial effects when applied in several animal models of heart infarct. Moreover, we very recently reported that Epo has protective effects on gentamicin-induced auditory hair loss (Monge et al. 2006).
Interestingly, all reports on Epo’s nonerythropoietic functions point towards a protective effect of this cytokine upon injury (mostly ischemia). We considered that Epo might also exert a physiological function, and indeed it does; Epo controls the hypoxic ventilatory response (HVR) in mice. During physiological hypoxia (as occurs at high altitude), pulmonary ventilation and arterial oxygen content are elevated by two complementary systems: the neuronal respiratory response (elaborated through the central and peripheral nervous system) leading to increased minute ventilation thereby increasing tissue oxygenation, and the renal-derived Epot that activates erythropoiesis in the bone marrow, thus augmenting the blood’s oxygen carrying capacity. Very recently we reported for the first time that these two systems do crosstalk via Epo (Soliz et al. 2005).
Carotid bodies are sensory organs whose stimulation by hypoxia activates a chemoreflex pathway. The sensory information is relayed to brainstem neurons that in turn modulate compensatory ventilatory adjustments. Carotid bodies are among the most vascularized organs in the body and are mainly stimulated by the decline of PaO2. As PC12 cells mimic carotid body type I cells, we hypothesized that these peripheral chemoreceptors might be also activated by Epo. To address this question we placed the animals into a plethysmograph (Fig. 1) and measured HVR in wild type mice upon injection of 2000 U/kg rhEpo. Epoinjected animals showed higher respiratory frequency but lower tidal volume than saline-injected controls when exposed to severe hypoxia (6% O2). These data suggest that Epo has an impact on carotid body cells, most probably by binding to the Epo receptor. We found a dense staining of EpoR in the carotid body, apparently localized within islets of chemosensitive cells. This observation implies that peripheral chemoreceptors can be activated by circulating Epo.

We also suspected that brain-derived Epo modulates ventilation by interacting with brainstem respiratory neurons. To test this hypothesis we used a transgenic mouse line (termed Tg21) that constitutively overexpresses human Epo in brain but shows normal Epo plasma levels (Wiessner et al. 2001). Tg21 mice showed improved ventilatory response to severe acute hypoxia and improved ventilatory acclimatization to chronic hypoxic exposure. Interestingly, following bilateral transection of the carotid sinus nerves that uncouple the brain from the carotid body (e.g chemodenervation), Tg21 mice adapted their ventilation to acute severe hypoxia while chemodenervated wild type (WT) animals developed a life-threatening apnoea (Fig. 2).
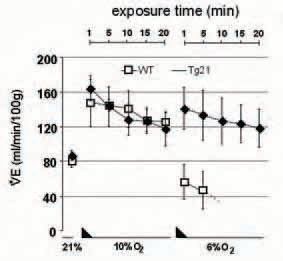
These results imply that Epo in the brain modulates ventilation. Immunohistochemical analysis revealed expression of the Epo receptor in the brainstem’s respiratory centres. Additionally, we also provided evidence that Epo modulates breathing control by alteration of catecholaminergic metabolism in brainstem. Taken together, these observations indicate that brain-derived Epo is a key player that modulates neural respiratory control by acting on the central nervous system.
In summary, we have demonstrated that high Epo levels in brainstem respiratory neurons improve ventilatory response and acclimatization to hypoxia. Furthermore, a link between Epo and respiratory control is provided through the peripheral nervous system by the carotid bodies that express the Epo receptor. In other words, Epo is a multifunctional signalling molecule physiologically acting on red blood cell production and lung ventilation to improve the net oxygen inflow to tissues and cells.
In considering the millennial population of high altitude dwellers, an obvious question arises regarding the impact of plasma and cerebral Epo in the ventilatory adaptation to high altitude. Do high altitude inhabitants have higher concentration of Epo in neural tissue? Does systemic Epo in high altitude dwellers enhance stimulation of carotid body functions? In keeping with this, high altitude de-adaptation syndrome is inherent in highlander populations. In patients suffering from so-called chronic mountain sickness, hypoventilation is associated with excessive erythrocytosis, suggesting that the connection between ventilation and Epo signals is failing. Consequently, the question about the implication of neural and plasma Epo in chronic mountain sickness has to be answered.
Finally, Epo is on the doping list because it augments the blood’s oxygen carrying capacity and thus the oxygen delivery to tissue. Considering that an increased level of plasma Epo stimulates carotid bodies under hypoxic conditions, it is tempting to speculate that Epo-mediated improved performance in athletes is also partially due to enhanced ventilatory and/or sympathetic capacity.
Obviously, Epo will keep us all very busy, as the ‘old’ drug has many ‘new’ functions that await detailed investigation.
References
Ehrenreich H, Hasselblatt M, Dembowski C, Cepek L, Lewczuk P, Stiefel M, Rustenbeck HH, Breiter N, Jacob S, Knerlich F, Bohn M, Poser W, Ruther E, Kochen M, Gefeller O, Gleiter C, Wessel TC, De Ryck M, Itri L, Prange H, Cerami A, Brines M & Siren AL (2002). Erythropoietin therapy for acute stroke is both safe and beneficial. Mol Med 8, 495-505.
Gassmann M, Heinicke K, Soliz J & Ogunshola OO (2003). Nonerythroid functions of erythropoietin. Adv Exp Med Biol 543, 323-330.
Grimm C, Wenzel A, Groszer M, Mayser H, Seeliger M, Samardzija M, Bauer C, Gassmann M & Reme CE (2002). HIF-1-induced erythropoietin in the hypoxic retina protects against light-induced retinal degeneration. Nat Med 8, 718-724.
Monge A, Nagy I, Bonabi S, Schmid S, Gassmann M & Bodmer D (2006). The effect of erythropoietin on gentamicin-induced auditory hair cell loss. Laryngoscopy 116, 312-316.
Sasaki R, Masuda S & Nagao M (2001). Pleiotropic functions and tissue-specific expression of erythropoietin. News Physiol Sci 16, 110-113.
Soliz J, Joseph V, Soulage C, Becskei C, Vogel J, Pequignot JM, Ogunshola O & Gassmann M (2005). Erythropoietin regulates hypoxic ventilation in mice by interacting with brainstem and carotid bodies. J Physiol 568, 559-571.
Wiessner C, Allegrini PR, Ekatodramis D, Jewell UR, Stallmach T & Gassmann M (2001). Increased cerebral infarct volumes in polyglobulic mice overexpressing erythropoietin. J Cereb Blood Flow Metab 21, 857-864.
