Physiology News Magazine
Exercise-induced lipid mobilization in humans: the role of catecholamines revisited
Bidirectional communication exists between the central nervous system and white adipose tissue. Here we discuss the relative contribution of the various factors involved in exercise-induced lipid mobilization in humans, reassessing the role of catecholamines
Features
Exercise-induced lipid mobilization in humans: the role of catecholamines revisited
Bidirectional communication exists between the central nervous system and white adipose tissue. Here we discuss the relative contribution of the various factors involved in exercise-induced lipid mobilization in humans, reassessing the role of catecholamines
Features
Max Lafontan (1) and Vladimir Stich (2)
1: Inserm Unit 858, Institut de Médecine Moléculaire de Rangueil, BP84225 31432 Toulouse cedex 4, France
2: Inserm Franco-Czech Laboratory for Clinical Research on Obesity and Department of Sport Medicine, Third Faculty of Medicine, Charles University, Prague, Czech Republic
https://doi.org/10.36866/pn.78.24

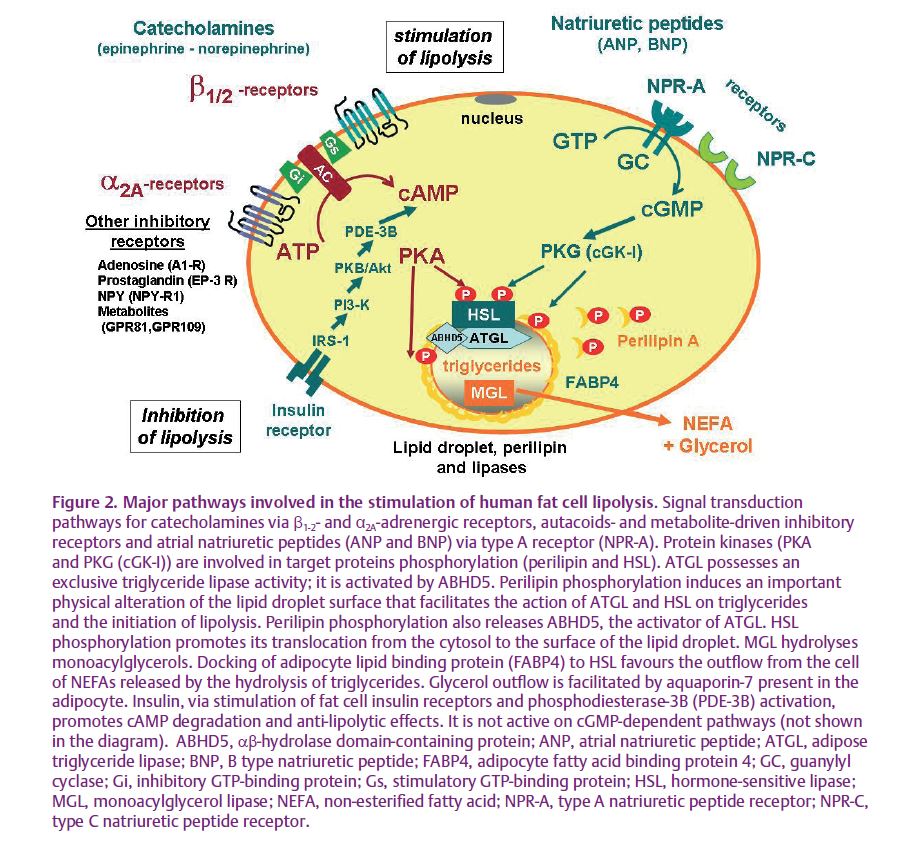
A number of questions concerning the regulation of lipid mobilization (i.e. hydrolysis/lipolysis of triglycerides stored in the adipocytes) have been mostly investigated in rodents, the adipose tissue of which clearly differs from that of humans. Lipolysis is such an important metabolic event that a number of redundant factors and pathways contribute to its physiological regulation (Lafontan & Langin, 2009) (Fig. 1). The debated questions concerning the role of the sympathetic nervous system in the control of white adipose tissue lipolysis have recently been reviewed by Bartness et al. (2009). White adipose tissue is innervated by the sympathetic nervous system but at very low levels of innervation of adipocytes. Moreover, the sympathetic nervous system drive to the various body fat deposits is not homogenous. In humans, up until recently, the major regulators of lipid mobilization (i.e. hydrolysis of triglycerides stored in the adipocytes) were considered to be the catecholamines (adrenaline (ephinephrine) and noradrenaline (norepinephrine)) as the stimulators of lipolysis and insulin for its inhibition.

In humans, the unquestionable impact of both catecholamines on lipid mobilization is observed when they are infused intravenously. However, the relative contribution of both amines in the physiological Vladimir Stich (left) and Max Lafontan control of lipid mobilization during physical exercise has been less convincingly established. Exercise is an excellent physiological challenge to promote sympathetic nervous system activation; there is no doubt that it contributes to the control of lipid mobilization, since plasma levels of adrenaline and noradrenaline are increased during exercise. They stimulate both fat cell β1-2- and α2A-adrenergic receptors, which enhance and inhibit lipolysis, respectively. In fact, the simultaneous activation of both receptors modulates the intracellular cAMP concentration, which activates cAMP-dependent protein kinase, leading to the phosphorylation and activation of the hormone-sensitive lipase (Lafontan & Langin, 2009). Growth hormone (GH) and other putative lipolytic candidates (i.e. natriuretic peptides or IL-6) have also been suspected to contribute (Fig. 1). In addition, exercise is also well known to promote inhibition of insulin release that is related to sympathetic nervous system-mediated inhibition of insulin release at the level of the pancreatic β-cells. Suppression of the anti-lipolytic effect of insulin is important to enhance the lipolytic activity of the fat cells. Nevertheless, contrary to former beliefs, studies have questioned the importance of the contribution of catecholamines. It has been shown, at low and moderate intensities of exercise, that under an oral β-adrenergic receptor blockade associated with a local perfusion of propranolol, the exercise-induced increase of lipolysis was diminished but not completely inhibited (Moro et al. 2004). Based on previous studies demonstrating the lipolytic role of natriuretic peptides (Sengenes et al. 2000) (Fig. 2), the residual lipolysis remaining under full β-adrenergic receptor blockade was attributed to these peptides. In fact, plasma levels of natriuretic peptides increase during an acute bout of physical exercise, and an enhanced release of these peptides occurs when exercise is performed under oral β-adrenergic receptor blockade (Moro et al. 2004). Further studies have shown that natriuretic peptides are physiological contributors of exercise-induced lipid mobilization in various situations in humans, though this system is not operative in rodents and dogs (Lafontan et al. 2008).
The relative contribution of the various factors involved in the control of exercise-induced lipid mobilization still remains an open and intricate challenge. A recent study investigating this complex question has allowed the delineation of the relative contribution of adrenaline and noradrenaline in the control of exercise-induced lipid mobilization in man to be elucidated (de Glisezinski et al. 2009), and revealed the importance of adrenaline instead of noradrenaline.
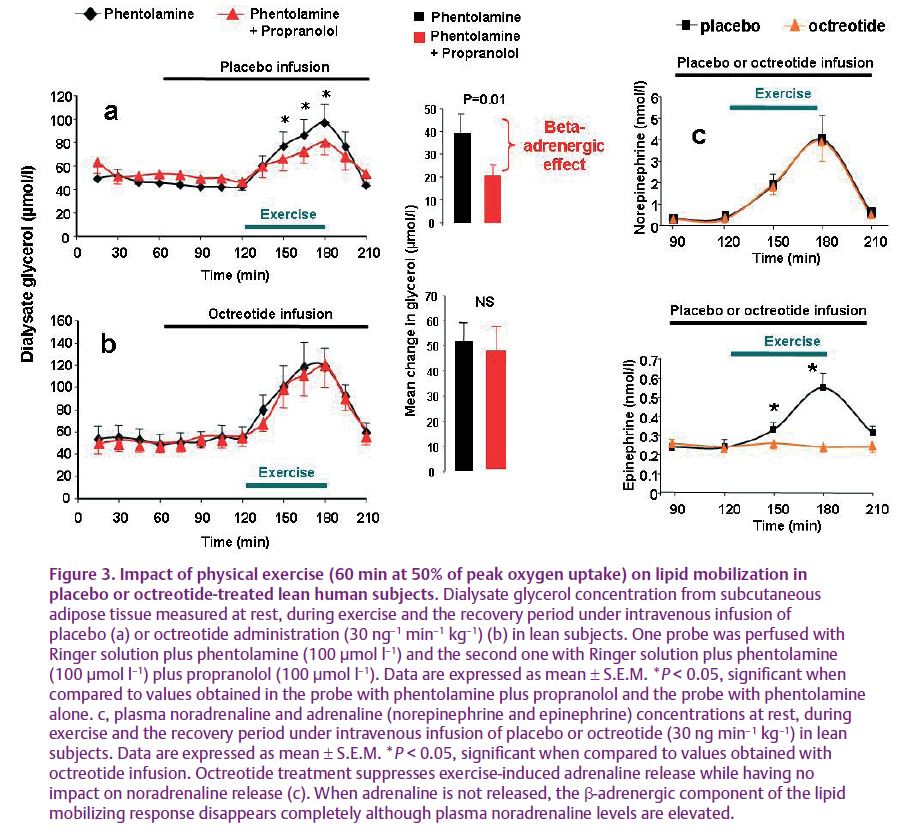
A pharmacological strategy was used to support this physiological approach and eliminate the intervention of some factors. Firstly, the catecholaminergic responsiveness was studied when insulin and GH secretions were suppressed. An intravenous infusion of the somatostatin analogue, octreotide, was given before, during and after exercise; it potently inhibited insulin and GH secretion during exercise. In addition, the noticeable point was that adrenaline secretion was also blocked by octreotide treatment while the exercise-induced increase in noradrenaline release was not modified (Fig. 3c). Thus, octreotide administration provides a unique condition whereby the impact of adrenaline and noradrenaline on lipolysis could be dissociated. Experiments were performed on healthy lean young men, fasted overnight and performing exercise bouts over 60 min at 50% of their peak oxygen uptake during placebo or octreotide administration.
Microdialysis was used to monitor local lipid mobilization (i.e. glycerol release) in subcutaneous adipose tissue during the exercise. It is a well-recognized method for mechanistic explorations of adipose tissue responsiveness in vivo.
Local blockade of fat cell β- and α2A-adrenergic receptors was carried out in situ by direct perfusion of the β- (i.e. propranolol) and α2A- (i.e. phentolamine) antagonists through the microdialysis probes to investigate the part played by catecholamines during exercise. The physiological stimulation of adipocyte α2-adrenergic receptors during exercise-induced sympathetic nervous system activation contributes to the blunted lipolysis. Phentolamine suppresses the blunting effect of α2A-adrenergic receptor stimulation. The blockade of α2A-adrenergic receptors removes the lipolysis-inhibiting part of the catecholamine action during exercise (Stich et al. 2000). If, in addition to that, when the local β-adrenergic receptor blockade was performed, the exercise-induced lipolysis was reduced in control (Fig. 3a) but not in the octreotide condition (Fig. 3c). This suggests that the β-adrenergic stimulation of lipolysis during exercise is mediated by adrenaline but not noradrenaline. In fact, both plasma levels of adrenaline and noradrenaline ‘normally’ increase when physical exercise is performed in control conditions. Under octreotide infusion the exercise-induced increase of plasma adrenaline is completely blocked while noradrenaline increment persists (Fig. 3c).
Based on these results and on some previous studies (Stallknecht et al. 2001), it is suggested that it is plasma adrenaline rather than noradrenaline which is the main adrenergic factor involved in exercise-induced lipolysis, at least in human subcutaneous adipose tissue. It must be remembered that adrenaline is known to possess the highest affinity, when compared with noradrenaline, for β2- and α2A-adrenergic receptors, the major adrenergic receptors of the human adipocytes (Lafontan & Berlan, 1982). In addition, the persistence of lipid mobilization after the full blockade of fat cell adrenergic receptors suggests that, as previously mentioned, it is the natriuretic peptides which constitute the lipid-mobilizing factor responsible for the exercise-induced lipid mobilization in subcutaneous adipose tissue (Lafontan et al. 2008). This study is focused on the importance of circulating factors (i.e. adrenaline, insulin and natriuretic peptides) in the control of exercise-induced lipid mobilization in human subcutaneous adipose tissue. The sympathetic drive to white adipose tissue differs according to fat deposits and so the importance of innervation-mediated effects versus adrenaline effects could produce some variability. Nevertheless, coming before the hormonal hypothesis, both the denervation approaches and direct sympathetic nerve stimulation support a role for sympathetic innervation in the initiation of lipolysis in rodents and humans. We must keep in mind that these procedures have a tendency to affect both sympathetic nerve fibres but also sensory fibres present in the nerves that could interfere in the control of energy stores (Bartness et al. 2009).
References
Bartness TJ, Shrestha YB, Vaughan CH, Schwartz GJ & Song CK (2009). Sensory and sympathetic nervous system control of white adipose tissue lipolysis. Mol Cell Endocrinol doi:10.1016/j.mce.2009.08.031
de Glisezinski I, Larrouy D, Bajzova M, Koppo K, Polak J, Berlan M, Bulow J, Langin D, Marques MA, Crampes F, Lafontan M & Stich V (2009). Adrenaline but not noradrenaline is a determinant of exercise-induced lipid mobilization in human subcutaneous adipose tissue. J Physiol 587, 3393–3404. http://jp.physoc.org/content/587/13/3393.long
Lafontan M & Berlan M (1982). Characterization of physiological agonist selectivity of human fat cell α2-adrenoceptors: adrenaline is the major stimulant of the α2-adrenoceptors. Eur J Pharmacol 82, 107–111.
Lafontan M & Langin D (2009). Lipolysis and lipid mobilization in human adipose tissue. Prog Lipid Res 48, 275–297.
Lafontan M, Moro C, Berlan M, Crampes F, Sengenes C & Galitzky J (2008). Control of lipolysis by natriuretic peptides and cyclic GMP. Trends Endocrinology Metab 19, 130–137.
Moro C, Crampes F, Sengenes C, De Glisezinski I, Galitzky J, Thalamas C, Lafontan M & Berlan M (2004). Atrial natriuretic peptide contributes to physiological control of lipid mobilization in humans. FASEB J 18, 908–910.
Sengenes C, Berlan M, De Glisezinski I, Lafontan M & Galitzky J (2000). Natriuretic peptides: a new lipolytic pathway in human adipocytes. FASEB J 14, 1345–1351.
Stallknecht B, Lorentsen J, Enevoldsen LH, Bulow J, Biering-Sorensen F, Galbo H & Kjaer M (2001). Role of the sympathoadrenergic system in adipose tissue metabolism during exercise in humans. J Physiol 536, 283–294.
Stich V, De Glisezinski I, Crampes F, Hejnova J, Cottet-Emard JM, Galitzky J, Lafontan M, Riviere D & Berlan M (2000). Activation of α2-adrenergic receptors impairs exercise-induced lipolysis in SCAT of obese subjects. Am J Physiol Regul Integr Comp Physiol 279, R499–R504.