
Physiology News Magazine
Exploring connections between the cerebellum and motor cortex in humans
Animal studies have demonstrated that the cerebellum influences both inhibitory and excitatory neurons in the motor cortex. Daskalakis and colleagues use transcranial magnetic stimulation to elucidate such connectivity in healthy human subjects
Features
Exploring connections between the cerebellum and motor cortex in humans
Animal studies have demonstrated that the cerebellum influences both inhibitory and excitatory neurons in the motor cortex. Daskalakis and colleagues use transcranial magnetic stimulation to elucidate such connectivity in healthy human subjects
Features
Z Jeff Daskalakis
Centre for Addiction and Mental Health, University of Toronto, Canada
Robert Chen
Toronto Western Research Institute, University of Toronto, Canada
https://doi.org/10.36866/pn.58.35

It is well known that both the motor cortex and the cerebellum are important structures involved in motor control. Previous animal and human studies had shown that Purkinje cells, output neurons of the cerebellar cortex, form inhibitory connections with the deep cerebellar nuclei (DCN), which have a disynaptic excitatory pathway through the ventral thalamus to the motor cortex. Clarifying the interaction between cerebellum and the motor cortex in healthy human subjects is important not only for understanding motor control, but also to help advance the pathophysiology of neurological and psychiatric diseases. For example, essential tremor can be treated by lesioning or high frequency stimulation of the cerebellar thalamus (Schuurman et al. 2000) and dysfunction of cerebellar-cortical connectivity has been proposed as a mechanism mediating schizophrenia.
In the article by Daskalakis et al. (Daskalakis et al. 2004), a unique approach involving a combination of stimulation pulses was used to evaluate the connectivity between the cerebellum and the motor cortex in human subjects. This approach involves delivering two transcranial magnetic stimuli (TMS), known as conditioning stimuli (CS), one to a distant cortical site such as the cerebellum, and one to a local cortical site over the motor cortex, prior to a single test stimulus (TS) to the motor cortex. The local CS stimulus has been shown to activate local inhibitory and excitatory cortical interneurons and, when preceded by a CS applied to distant cortical sites, can test how distant brain regions interact with local cortical interneurons to modulate cortical pyramidal cell output.
Cerebellar activation was achieved through TMS applied to the cerebellum with a double-cone coil (110 mm mean diameter). The coil was centered over the right cerebellar hemisphere 3 cm lateral to the inion on the line joining the inion and the external auditory meatus. The double-cone coil provides stronger magnetic field that penetrates deeper into the brain than standard flat figure-of-eight coils and previous studies showed that it activates cerebellar inhibitory neurons (Ugawa et al. 1995). Local motor cortical activation was achieved through TMS of the left motor cortex with a 7 cm figure of eight coil.
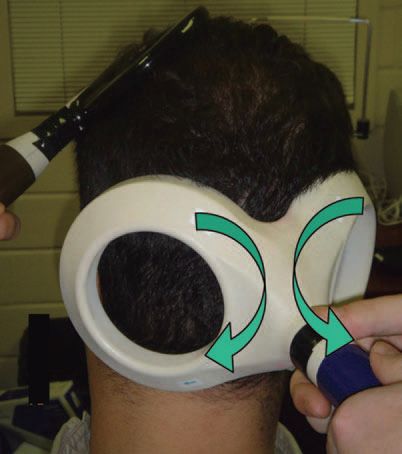
Three separate experiments were conducted to evaluate the connectivity between the cerebellum and motor cortex in humans. The first involved a controlled manipulation of TS intensities on several inhibitory and excitatory TMS paradigms. These include cerebellar inhibition (CBI), short interval cortical inhibition (SICI), long interval cortical inhibition (LICI) and intracortical facilitation (ICF). If these paradigms share common mechanisms, their profiles of response under conditions of controlled manipulation should be similar. Second, connectivity can be explored by examining the impact of one inhibitory/excitatory phenomenon on the other by applying them together. Here, the interaction between CBI and SICI is tested when a cerebellar conditioning stimulus precedes a cortical conditioning stimulus by 3 ms which precedes a suprathreshold TS by 2 ms. In this way, the effect of cerebellar activation on cortical interneurons may be examined. Finally, the interaction between CBI and LICI is tested when a cortical conditioning stimulus precedes a cerebellar conditioning stimulus by 95 ms which precedes a suprathreshold TS by 5 ms. Here too, this triple stimulus approach allows one to test the interaction between these two inhibitory paradigms. Moreover, as it has been suggested previously that SICI is mediated primarily by inhibitory interneurons producing fast IPSPs due to GABAA receptors, whereas LICI is mediated primarily by inhibitory interneurons producing slow IPSPs due to GABAB receptors, this approach allows us to test the effects of cerebellar inhibition on these two different populations of inhibitory interneurons.
In experiment 1 we found that increasing TS intensities resulted in less LICI, CBI and ICF but greater SICI. In experiment 2, SICI was reduced and ICF was increased in the presence of CBI. It was speculated that CBI may decrease SICI through reduced thalamocortical facilitation of cortical inhibitory interneurons mediating SICI. This connectivity may be important for the dynamic focusing of motor output (Shinoda et al. 1993). In experiment 3, it appears that CBI and LICI interact to produce decreased inhibition in the motor cortex.
It was suggested that reduced inhibition was mediated by one of the following mechanisms:
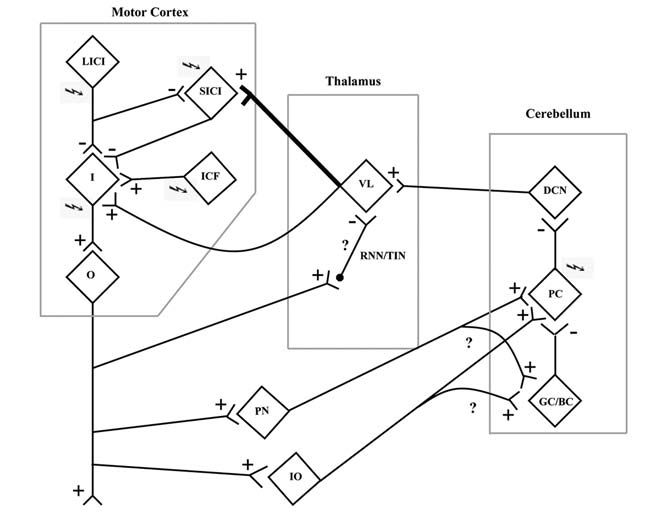
- Disruption of the cerebellothalamocortical inhibitory pathway at the level of the motor thalamus. Animal studies have shown that cortical stimulation results in activation of reticular nuclei neurons and thalamic inhibitory neurons, which, in turn, inhibit the cerebellothalamocortical pathway (Ando et al. 1995) (Fig. 2).
- Motor cortex stimulation from the CS pulse influences the cerebellar cortex through activation of the mossy fibers that come from the pontine nuclei via the corticopontocerebellar pathway. Activation of this pathway may inhibit Purkinje cells through activation of inhibitory Golgi and basket cells in the cerebellar cortex. Delivery of the cerebellar CS in the presence of inhibited Purkinje cells would produce the loss of CBI seen in this experiment.
- Yet another possibility is that motor cortex stimulation results in decreased Purkinje cell inhibitory output through activation of the inferior olive (Schwarz & Welsh, 2001). Here, the collaterals of climbing fibers from the inferior olive also innervate the Golgi and basket cells leading to suppression of Purkinje cells.
Our findings suggest that cerebellar stimulation results in changes to both inhibitory and excitatory neurons in the human motor cortex, to our knowledge one of the first times that such connectivity has been demonstrated in healthy human subjects.
References
Ando N, Izawa Y & Shinoda Y (1995). Relative contributions of thalamic reticular nucleus neurons and intrinsic interneurons to inhibition of thalamic neurons projecting to the motor cortex. J Neurophysiol 73, 2470-2485.
Daskalakis ZJ, Paradiso GO, Christensen BK, Fitzgerald PB, Gunraj C & Chen R (2004). Exploring the connectivity between the cerebellum and motor cortex in humans. J Physiol 557, 689-700.
Sanger TD, Garg RR & Chen R (2001). Interactions between two different inhibitory systems in the human motor cortex. J Physiol 530, 307-317.
Schuurman PR, Bosch DA, Bossuyt PM, Bonsel GJ, van Someren EJ, de Bie RM, Merkus MP & Speelman JD (2000). A comparison of continuous thalamic stimulation and thalamotomy for suppression of severe tremor. N Engl J Med 342, 461-468.
Schwarz C & Welsh JP (2001). Dynamic modulation of mossy fiber system throughput by inferior olive synchrony: a multielectrode study of cerebellar cortex activated by motor cortex. J Neurophysiol 86, 2489-2504.
Shinoda Y, Kakei S, Futami T & Wannier T (1993). Thalamocortical organization in the cerebello-thalamo-cortical system. Cereb. Cortex 3, 421-429.
Ugawa Y, Uesaka Y, Terao Y, Hanajima R & Kanazawa I (1995). Magnetic stimulation over the cerebellum in humans. Ann Neurol 37, 703-713.
