
Physiology News Magazine
‘Fibroblast-like cells’ mediate purinergic neurotransmission in gastrointestinal smooth muscles
Fibroblast-like cells are abundant in smooth muscle tissues, but little is known about the functional role of these cells in normal tissues and their fate in pathophysiological conditions. New genetic animal models now make it possible to isolate fibroblasts from smooth muscle tissues, and physiological and molecular analyses are beginning to reveal the physiological role of these cells in health and disease.
Features
‘Fibroblast-like cells’ mediate purinergic neurotransmission in gastrointestinal smooth muscles
Fibroblast-like cells are abundant in smooth muscle tissues, but little is known about the functional role of these cells in normal tissues and their fate in pathophysiological conditions. New genetic animal models now make it possible to isolate fibroblasts from smooth muscle tissues, and physiological and molecular analyses are beginning to reveal the physiological role of these cells in health and disease.
Features
Kenton M. Sanders and Masaaki Kurahashi
Department of Physiology and Cell Biology, University of Nevada School of Medicine, Reno, NV 89557, USA
https://doi.org/10.36866/pn.85.29

Morphologists have described fibroblasts in smooth muscle tissues for many years, and it has been assumed that these cells are involved in construction and maintenance of the extracellular matrix and in other housekeeping duties. In gastrointestinal (GI) muscles, fibroblasts are not randomly distributed, as might be the case if the cells were involved only in maintenance of the extracellular matrix. In fact, the distribution of fibroblasts is analogous to another type of interstitial cell, interstitial cells of Cajal (ICC), which have important functions such as pacemaker activity, propagation of electrical slow waves, mediation of post-junctional responses to enteric motor neurons, and mediation of responses to stretch (Sanders et al. 2010). A well-ordered distribution often predicts specific roles for cells in the function of a tissue.
Morphology and ultrastructure of fibroblast cells in GI muscles
Initial investigations of interstitial cells of the GI tunica muscularis by Terumasa Komuro with scanning electron microscopy showed flattened cells with many processes in the region of the myenteric plexus, but it was difficult to distinguish between ICC and fibroblast cells with this technique (Komuro, 1989). Transmission electron microscopy (TEM) provided ultrastructural detail and allowed Komuro and others to observe distinct structural differences between ICC and fibroblasts. TEM became the best technique for identifying these cell types in smooth muscle tissues. Well-developed rough endoplasmic reticulum, Golgi and the absence of a basal lamina are among distinguishing characteristics of fibroblasts. These cells were also clustered around neurons with distances of as little as 50 nm between nerve varicosities and fibroblasts. Fibroblasts also formed gap junctions with each other and with smooth muscle cells. In fact, serial sections showed that fibroblasts formed an electrical bridge between the circular and longitudinal muscle layers, which may explain electrical coupling and coordination between muscle layers.
Expression of ion channels mediating enteric neurotransmission in fibroblast cells
Fibroblasts were also found to express ion channels that could mediate post-junctional responses to purine neurotransmitters (i.e. small-conductance Ca2+-activated K+ channels, SK3). Klemm & Lang (2002) observed SK3 immunoreactivity in the guinea-pig GI tract in cells with distributions similar to ICC. They speculated that SK3+ cells might mediate purinergic responses in GI muscles. SK3+ cells were shown to be CD34+/Kit– fibroblast-like cells in human and murine GI muscles (Vanderwinden et al. 2002). A confusing issue from molecular expression studies, however, was that smooth muscle cells express SK2 channels (Klemm & Lang, 2002), another isoform of small-conductance Ca2+-activated K+ channels. Thus, while opening the door to the possibility that cells besides smooth muscle cells might mediate purinergic neurotransmission, expression studies were not able to determine with certainty the cells responsible for inhibitory neural regulation.
New tools to study fibroblast cells
We were intrigued by the proximity of fibroblast-like cells to enteric nerve terminals and their abundant expression of SK3 channels because we had previously documented a role for ICC in enteric motor neurotransmission (Sanders et al. 2010). Until recently, we lacked tools needed to study these cells selectively. Satoshi Iino and his collaborators are credited with providing a key observation that facilitated investigation of the fibroblast cells by showing that these are labelled in GI muscles specifically by antibodies to PDGFRα (Iino et al. 2009). PDGFRα+ cells in the tunica muscularis are a population of cells quite distinct from ICC, because double labelling with PDGFRα and Kit antibodies does not co-localize. Iino and colleagues used immuno-electron microscopy to show that PDGFRα+ cells were distinct from ICC, closely associated with neurons in muscle bundles, and possessed ultrastructural features that identified them as fibroblast-like cells (Iino et al. 2009). PDGFRα immuno-labelling highlighted the organized anatomical localization of these cells in tissue niches shared by enteric ganglia, processes of motor neurons, tissue macrophages and interstitial cells of Cajal and confirmed expression of SK3 by PDGFRα+ cells. In fact, so similar is the distribution of PDGFRα+ cells and ICC, we chose to refer to these cells with the same nomenclature used by most investigators to distinguish sub-classes of ICC (i.e. cells with multiple processes found in the region of the myenteric plexus, but outside ganglia, are termed PDGFRα-MY and intramuscular cells found within muscle bundles in close association with the terminals of enteric motor neurons are termed PDGFRα-IM).
Iino’s immunohistochemical techniques (Iino et al. 2009) also provided unique opportunities to better understand the physiology of these cells. To accomplish this, we used a transgenic animal that was engineered to utilize the endogenous, cell-specific promoters for Pdgfra to drive expression of
a histone–eGFP fusion protein. Thus, cells that normally express PDGFRα display bright green nuclei due to the constitutive expression of eGFP (Fig. 1A–C). These animals provided an opportunity to collect identifiable PDGFRα+ cells for molecular expression and physiological studies. When GI muscles from these animals were dispersed with proteolytic enzymes, small round cells with 6.0 pF cell capacitance were identified in the mixed cell populations (Kurahashi et al. 2011). It should be noted that PDGFRα+ cells are likely to be prevalent in enzymatic dispersions of all smooth muscle tissues, and these cells may be a major component of preparations often referred to (possibly in a manner that is markedly too cavalier) as ‘cultured smooth muscle cells’ and used widely as models of smooth muscle growth, plasticity and cell signalling.
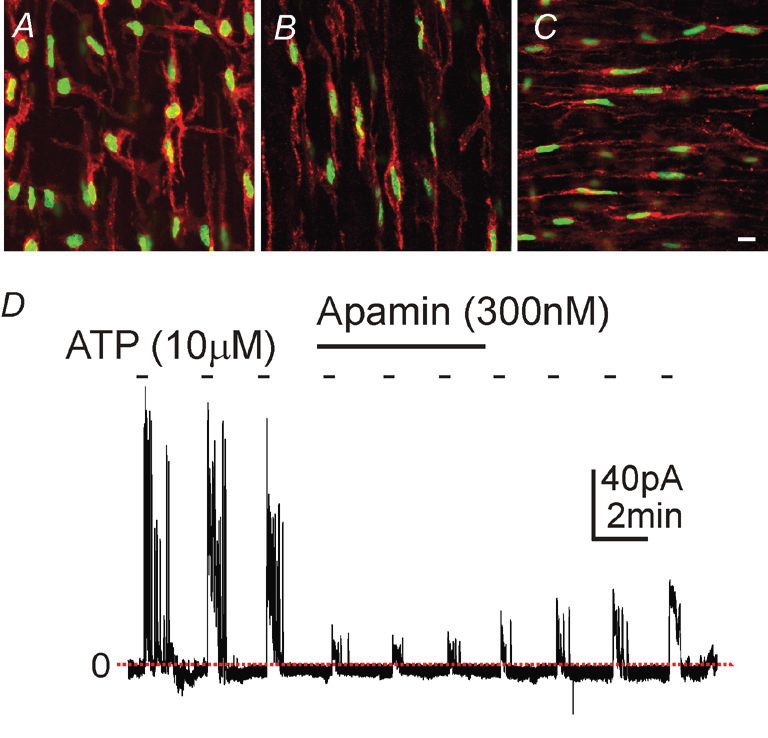
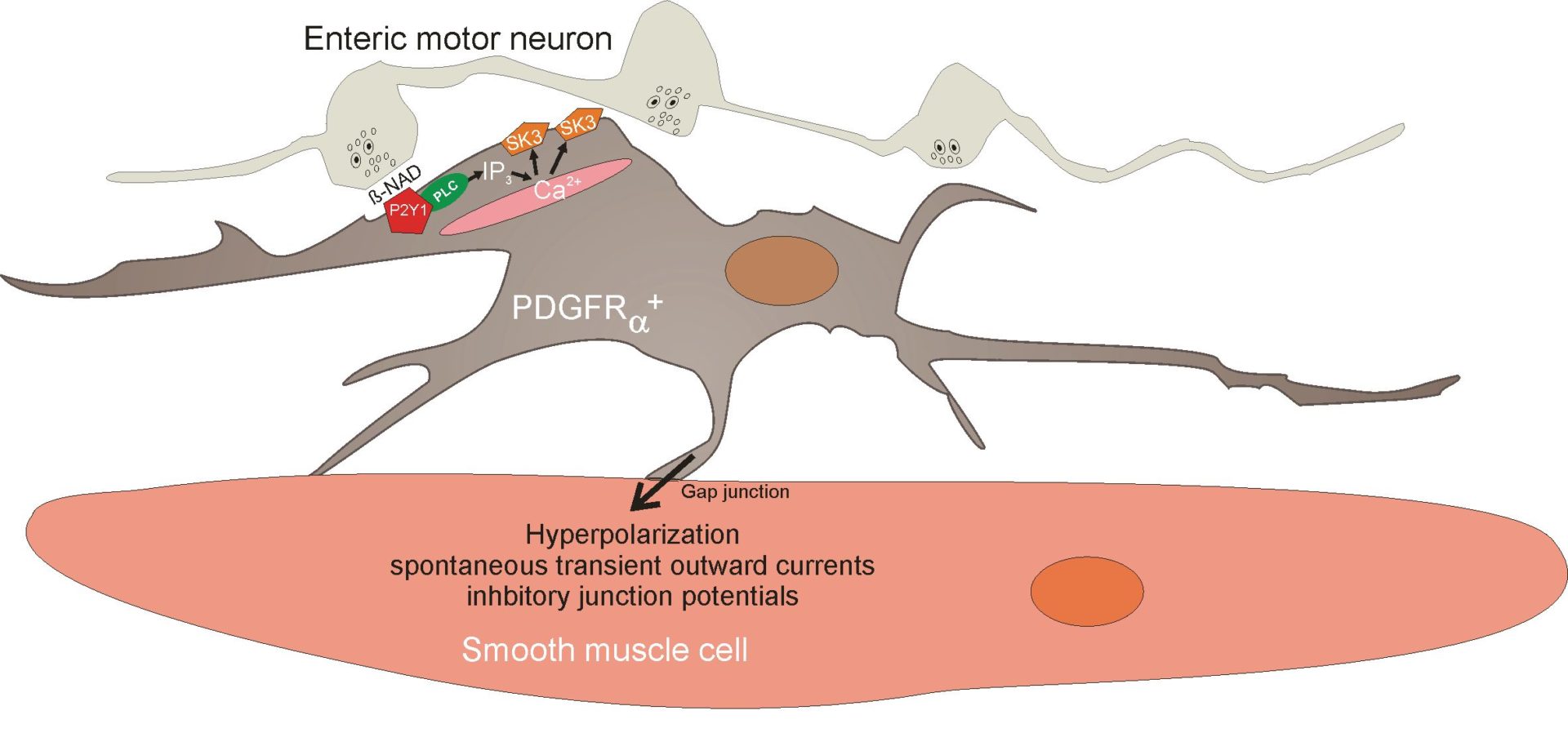
A new player in enteric motor neurotransmission
A consistent observation about fibroblast cells in GI muscles is close association with enteric motor neurons. PDGFRα+ cells also form gap junctions with neighbouring smooth muscle cells, thus creating a low-resistance electrical pathway between these cells and the smooth muscle syncytium. Since PDGFRα+ cells express SK3 channels and a component of post-junctional responses to inhibitory nerve stimulation is blocked by apamin, a potent inhibitor of SK3 channels, we tested the hypothesis that PDGFRα+ cells may mediate purinergic enteric inhibitory responses in GI muscles. Pharmacological studies have also shown that P2Y1 receptors mediate post-junctional purinergic responses in GI muscles, so we tested and confirmed expression of P2Y1 receptors and SK3 channels by PDGFRα+ cells (Kurahashi et al. 2011). Under whole-cell voltage-clamp conditions, PDGFRα+ cells generated large-amplitude, Ca2+-dependent outward currents in response to P2Y1 agonists (Fig. 1D), including β-NAD, a novel purinergic neurotransmitter in the gut (Mutafova-Yambolieva et al. 2007). Excised patches of membrane from PDGFRα+ cell exhibited 10 pS, Ca2+-sensitive K+ channels consistent with the expression of SK3 by these cells. Under current clamp, the cells generated sharp hyperpolarization responses consistent with the purinergic inhibitory junction potential. Under similar conditions, smooth muscle cells from the same tissues generated little outward current under voltage clamp and net small depolarizations in current clamp. Opening of apamin-sensitive K+ channels (i.e. SK channels) and a rapid hyperpolarization response is a hallmark of purinergic neurotransmission in the gut. Our data demonstrated that smooth muscle cells are incapable of generating purinergic inhibitory junction potentials in the GI tract, but the apparatus to generate hyperpolarization responses is clearly manifest in PDGFRα+ cells that are electrically coupled to smooth muscle cells. It should also be noted that many PDGFRα+ cells generated spontaneous transient outward currents (STOCs) that were apamin sensitive, suggesting that these cells may also contribute to the resting potentials of GI muscles independent of neural input. Thus, it appears that PDGFRα+ cells have at least two major functions in regulating GI motor behaviour (Fig. 2): (i) binding and transduction of purinergic enteric inhibitory neurotransmitter and (ii) regulation of resting membrane potential and control of
excitability of the smooth muscle/ICC/PDGFRα+ cell syncytium.
References
Iino S, Horiguchi K, Horiguchi S & Nojyo Y (2009). c-Kit-negative fibroblast-like cells express platelet-derived growth factor receptor alpha in the murine gastrointestinal musculature. Histochem Cell Biol 131, 691–702.
Klemm MF & Lang RJ (2002). Distribution of Ca2+-activated K+ channel (SK2 and SK3) immunoreactivity in intestinal smooth muscles of the guinea-pig. Clin Exp Pharmacol Physiol 29, 18–25.
Komuro T (1989). Three-dimensional observation of the fibroblast-like cells associated with the rat myenteric plexus, with special reference to the interstitial cells of Cajal. Cell Tissue Res 255, 343–351.
Kurahashi M, Zheng H, Dwyer L, Ward SM, Don Koh S & Sanders KM (2011). A functional role for the ‘fibroblast-like cells’ in gastrointestinal smooth muscles. J Physiol 589, 697–710. http://jp.physoc.org/content/589/3/697.long
Mutafova-Yambolieva VN, Hwang SJ, Hao X, Chen H, Zhu MX, Wood JD, Ward SM & Sanders KM (2007). β-Nicotinamide adenine dinucleotide is an inhibitory neurotransmitter in visceral smooth muscle. Proc Natl Acad Sci U S A 104, 16359–16364.
Sanders KM, Hwang SJ & Ward SM (2010). Neuroeffector apparatus in gastrointestinal smooth muscle organs. J Physiol 588, 4621–4639.
Vanderwinden JM, Rumessen JJ, de Kerchove d’Exaerde A Jr, Gillard K, Panthier JJ, de Laet MH & Schiffmann SN (2002). Kit-negative fibroblast-like cells expressing SK3, a Ca2+-activated K+ channel, in the gut musculature in health and disease. Cell Tissue Res 310, 349–358.
Acknowledgements
Studies of PDGFRα+ cells in the authors’ laboratories are supported by a Pilot Project Grant from the University of Nevada and Core Facilities funded by the National Institute of Diabetes and Digestive and Kidney Diseases P01 DK41315 to KMS.
