
Physiology News Magazine
Fishing for O2 chemoreceptors in vertebrates
Despite phylogenetic differences in modes of breathing, mammalian and aquatic vertebrates appear to utilize similar O2-sensing pathways
Features
Fishing for O2 chemoreceptors in vertebrates
Despite phylogenetic differences in modes of breathing, mammalian and aquatic vertebrates appear to utilize similar O2-sensing pathways
Features
Michael G. Jonz (1) & Colin A. Nurse (2)
1: Department of Physiology and Biophysics, Dalhousie University, Halifax, Canada
2: Department of Biology, McMaster University, Hamilton, Canada
https://doi.org/10.36866/pn.58.39

Maintenance of arterial O2 tension by respiratory regulation is of fundamental importance for animal survival and adaptation to hypoxia (low O2). Detection of hypoxia occurs at the cellular level by specialized O2-sensitive cells that initiate local or centrally-mediated physiological responses, such as changes in ventilation and heart rate. In mammals, these include type I chemoreceptors of the carotid body (CB; the primary O2-sensing organ), pulmonary neuroepithelial bodies (NEBs), adrenal chromaffin cells, and arterial smooth muscle cells. A common mechanism for detecting O2 levels in these cells involves regulation of K+ channel activity during chemotransduction (López-Barneo et al. 2001). In CB type I cells for example, hypoxia causes a receptor potential, due to inhibition of K+ channels that are open at rest, leading to voltage-gated Ca2+ entry and release of neurotransmitters that activate sensory pathways. Despite the similar responses to hypoxia in all vertebrates, characterization of O2- chemoreception at the cellular level has traditionally been confined to airbreathing mammals. Yet, it might be expected that the basic cellular mechanisms arose earlier, perhaps in water-breathing vertebrates that rely on gills for ventilation and regulation of gas exchange.
Indeed, decades of research have implicated the gills as the common O2-chemoreceptive site in aquatic vertebrates, e.g. fish and larval amphibians (Burleson & Milsom, 2003). Interestingly, the gill or aortic arches in these animals share a common embryonic origin with O2sensitive carotid and aortic bodies of mammals. Moreover, the fish gill contains neuroepithelial cells (NECs) that have long been considered potential O2 chemoreceptors (Zaccone et al. 1997), which store the neurotransmitter serotonin (5-HT) and are morphologically similar to mammalian CB type I cells and pulmonary NEBs. The morphology and innervation pattern of NECs were recently described in the zebrafish gill (Jonz & Nurse, 2003). Although CB type I cells occur in clusters, rather than in isolation as in gill NECs, both cell types receive sensory innervation from the glossopharyngeal (IXth) nerve (Fig. 1A,B) and generate a receptor potential during exposure to acute hypoxia (Fig. 1C,D). The latter responses are attributable, at least in part, to inhibition of voltage-independent, quinidine-sensitive ‘leak’ K+ channels with biophysical and pharmacological properties of the two-pore domain, background K+ channel family (Buckler et al. 2000; Jonz et al. 2004). While the molecular identity of the O2-sensitive background K+ channel in zebrafish NECs awaits determination, in rat type I cells it appears to be related to the mammalian TASK-1 channel (Buckler et al. 2000). Thus, regulation of background K+ channels by hypoxia appears to be a fundamental mechanism that has been relatively conserved. Additionally, the O2-sensitivity of NECs (which are found on all gill arches) appears to confirm the hypothesis that throughout phylogenesis, and the later evolution of air-breathing, the distribution of peripheral O2 chemoreceptors was reduced from multiple dispersed sites (i.e. gills) in fish to a single primary location (i.e. carotid body), as found in mammals (Burleson & Milsom, 2003).
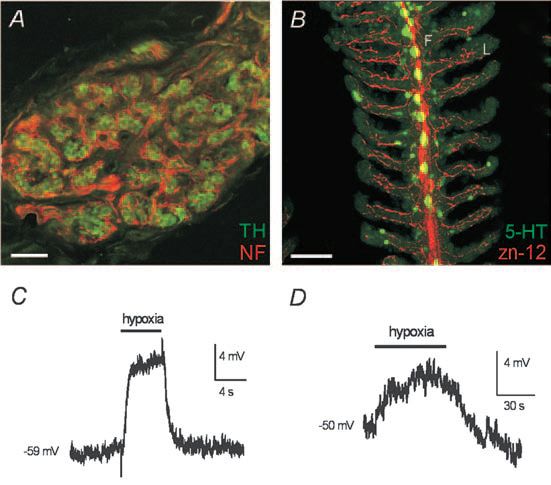
In spite of recent progress, the remaining steps in the cellular response to hypoxia, including the molecular identity of the O2 sensor, are still controversial. Current models suggest that the O2 sensor may be a plasma membrane or intracellular heme protein, capable of reversibly regulating ion channels directly or through a signalling cascade (López-Barneo et al. 2001; Williams et al. 2004). The popular redox hypothesis proposes that reactive oxygen species produced by NADPH oxidase or the mitochondrial electron transport chain regulate ion channel activity during hypoxia (López-Barneo et al. 2001). The recent identification of an O2-sensing mechanism in NECs of a model vertebrate, i.e. zebrafish (Jonz et al. 2004), and subsequent characterization of mutations that perturb this function, may lead to the elucidation of a general O2-sensing mechanism that arose early in vertebrate evolution.
Acknowledgements
We acknowledge the technical assistance of Cathy Vollmer and Min Zhang, and grant support from NSERC.
References
Buckler KJ, Williams BA & Honoré E (2000). An oxygen-, acid- and anaesthetic-sensitive TASK-like background potassium channel in rat arterial chemoreceptor cells. J Physiol 525, 135-142.
Burleson ML & Milsom WK (2003). Comparative aspects of O2 chemoreception: anatomy, physiology, and environmental adaptations. In Oxygen sensing: Responses and adaptation to hypoxia, eds. Lahiri S, Semenza GL & Prabhakar NR, pp. 685-707. Marcel Dekker, New York.
Jonz MG & Nurse CA (2003). Neuroepithelial cells and associated innervation of the zebrafish gill: a confocal immunofluorescence study. J Comp Neurol 461, 1-17.
Jonz MG, Fearon IM & Nurse CA (2004). Neuroepithelial oxygen chemoreceptors of the zebrafish gill. J Physiol 560, 737-752.
Williams SE, Wootton P, Mason HS, Bould J, Iles DE, Riccardi D, Peers C & Kemp PJ (2004). Hemoxygenase-2 is an oxygen sensor for a calcium-sensitive potassium channel. Science 306 (5704), 2093-2097.
López-Barneo J, Pardal R & Ortega-Sáenz P (2001). Cellular mechanisms of oxygen sensing. Annu Rev Physiol 63, 259-287.
Zaccone G, Fasulo S, Ainis L & Licata A (1997). Paraneurons in the gills and airways of fishes. Microsc Res Tech 37, 4-12.
