
Physiology News Magazine
Focus on Cardiac Mechano-Electric Feedback
Features
Focus on Cardiac Mechano-Electric Feedback
Features
Peter Kohl
University Lab of physiology, Oxford
https://doi.org/10.36866/pn.48.19
For the uninitiated, cardiac mechano- electric feedback (MEF) may seem a pretty dull topic: Isn’t it quite obvious, that the mechanical environment of the heart affects its electrophysiology?
Worse than that, for certain types of reviewers the topic simply doesn’t exist: Why are you wasting your time, stretching patch-clamped cells, merely to record artefacts?
Thankfully, a growing number of dedicated journal issues, invited reviews, and international meetings illustrate that the subject is alive and kicking. This year alone there will be an international workshop on Cardiac MEF & Arrhythmias at Oxford (http://mef.physiol.ox.ac.uk/), as well as dedicated sessions during NASPE’s annual meeting in San Diego and at CardioStim 2002 in Nice. It was very timely, therefore, that the Physiological Society chose to devote a 1-day symposium to cardiac MEF during its Leeds meeting.
So – what is this emm-eee-eff all about?
Clinical observations of mechanically induced heart rhythm disturbances date back more than 120 years, when first reports about sudden cardiac death after chest impact in the absence of structural damage were published (Meola, 1879). This condition, termed commotio cordis (Casper, 1857), has a long pedigree of targeted experimental research (Schlomka, 1934). More recently, helped by media reports on ‘unexplained’ sporting fatalities after chest impact during baseball or hockey (including coverage by popular TV series like ER), sudden cardiac death by commotio cordis is experiencing a renais- sance in professional and public interest (Maron et al, 2002).
Obviously, not all mechanical effects on heart rhythm are that dramatic. They may even be useful!
Ectopic beats, for example, that occur during cardiac catheterisation may be taken to indicate the final approach of the catheter tip to the heart. Moreover, in the days before lawyers determined clinical interventions, patients could be prevented from losing consciousness during Stokes-Adams attacks by ‘pre- cordial percussion’ – repeated thumps to the chest that triggered competent cardiac contractions – until normal rhythm recommenced (Schott, 1920).
Energy levels required for mechanical induction of cardiac excitation are comparatively low. Using a modified industrial stapling gun (!), pre-cordial impacts of 0.04–1.5 J were found to reliably trigger ectopic beats in healthy volunteers (Zoll et al, 1976).
These beneficial effects of cardiac MEF explain why in many countries, including the UK and US, precordial thumps are a sanctioned procedure for resuscitation after witnessed cardiac arrest. Does this really work? See Figure 1.
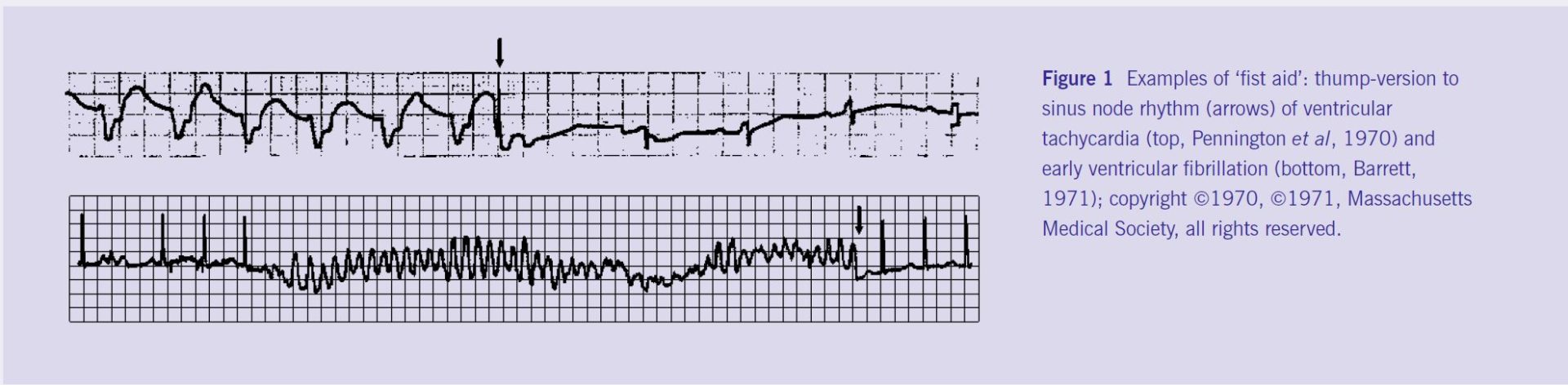
The wealth of case reports from patients (including heart transplant recipients) is complemented by decades of research, from in vivo work on mammals like man (Levine et al, 1988) and pig (Lab & Woollard, 1978), to ex situ studies in isolated hearts (Franz et al, 1992) or tissues (Deck, 1964), and in vitro experiments on single cells (White et al, 1995), membrane patches (Sachs et al, 1991), and proteins (Sukharev et al, 1994).
Basic research shows that many features of cardiac MEF may be reproduced in isolated cells and tissues. Key mechanisms include the activation of specialised mechano-sensitive ion channels (Craelius et al, 1988), mechanical modulation of second messengers like nitric oxide (Petroff et al, 2001) and calcium (Le Guennec et al, 1991), and the interaction of mechano-sensitive cells of both myocyte and non- myocyte origin (Kohl et al, 1999).
Nonetheless, apart from a number of praiseworthy exclusions from the rule, the ‘link-up’ of clinical studies and basic science has, thus far, been less than satisfactory (Taggart & Sutton, 1999). There are many reasons for this, including technical limitations such as the lack of specific drugs to probe cellular mechanisms of MEF in situ, but also geographical, linguistic and professional ‘divides’.
Some of these restrictions are currently being redressed. Thus, the identification of a first, highly selective peptide blocker of stretch-activated ion channels (Suchyna et al, 2000) has opened-up new perspectives in linking (sub-)cellular mechanisms of MEF to patho-physiological processes at higher levels of integration (Bode et al, 2001). Furthermore, extensive reviews of historical (mainly non-English) literature sources recovered much ‘lost’ insight from the past and helped to inform current research (Nesbitt et al, 2001). Finally, we are witnessing a more conscious approach towards bringing together physiologists, bioengineers, modellers and clinicians to discuss mechanisms and implications of cardiac MEF.
The Leeds meeting is set to continue this trend, which will – hopefully in the near future – make cardiac MEF a standard item in all mainstream physiology-textbooks (…dream on, chap!).


