
Physiology News Magazine
Future directions in cardiac and respiratory physiology
A Theme Leads’ report
Features
Future directions in cardiac and respiratory physiology
A Theme Leads’ report
Features
Andrew F. James
University of Bristol, UK
Sarah Calaghan
University of Leeds, UK
https://doi.org/10.36866/pn.110.26


The membership of the Cardiac and Respiratory Physiology Theme (417 Full and 1137 other Members of The Society in 2018) encompasses an enormously diverse range of interests and experimental approaches in cardio-respiratory physiology, from cellular through organ level to in vivo function. We have not attempted to identify all abstracts in this area over the last two years but have found a total of 238 communications describing work relevant to this theme (www.physoc.org/proceedings). They are classified on the website in categories which emphasise the breadth and depth of activity of the Theme, for example: ‘Cardiovascular, Respiratory and Autonomic Control’, ‘Heart & Cardiac Muscle’, ‘Respiratory Physiology’ and
‘Ion Channels’.
According to the World Health Organization (https://www.who.int/), cardiac and respiratory diseases remain the leading causes of death globally. Disease-focused research that may open novel therapeutic avenues for relatively large numbers of people can readily be justified to funding organisations. In terms of heart disease, death rates are falling in many developed countries, presumably because of improved healthcare arising from a better understanding of the disease process. For example, within the UK, deaths from heart disease in England and Wales have halved since 2001 and Alzheimer’s disease and dementia are now the leading causes of death (Patel, 2017).
This remarkable change in less than two decades likely reflects a range of factors, including a real fall in deaths attributable to heart disease per se, but also changes to the classification of causes of death (Patel, 2017). However, as shown in figures recently reported by the British Heart Foundation (https://www.bhf.org.uk/research/heart-statistics), recent improvements in acute treatment and therefore survival for major cardiovascular events (e.g. myocardial infarction), combined with increased longevity, means that the number of people living with heart failure is showing an upward trend. Overall, cardiac and respiratory disease remains a major contributor to the global burden of disease.
There are many avenues for future research within this theme to be explored, employing the vast array of techniques available to the 21st century physiologist. Here we have chosen to highlight recent advances in imaging and gene editing technologies which are fuelling major developments in the cardiac and respiratory fields.
In order to fully understand normal physiology and the impact of disease, we need to be able to describe cellular structures on a nanoscale, and advances in microscopy techniques are making this possible. For example, 3D electron microscopy approaches (tomography, serial block-face scanning) allow visualisation of complex cellular structures such as the cardiac myocyte t-tubules, invaginations of the sarcolemma that play a central role in excitation-contraction coupling in the heart (e.g. Pinali et al., 2017). Single molecule localisation microscopies such as dSTORM and DNA-PAINT (which uses hybridisation of DNA oligonucleotides to localise protein targets) can provide true molecular-scale resolution of proteins such as the ryanodine receptor (Jayasinghe et al., 2018). For more about these techniques, see Carl Harrison’s article on p. 36 of this issue. The ability
to directly correlate nano-anatomy with aspects of myocyte function will prove invaluable in understanding the process of cardiac disease.
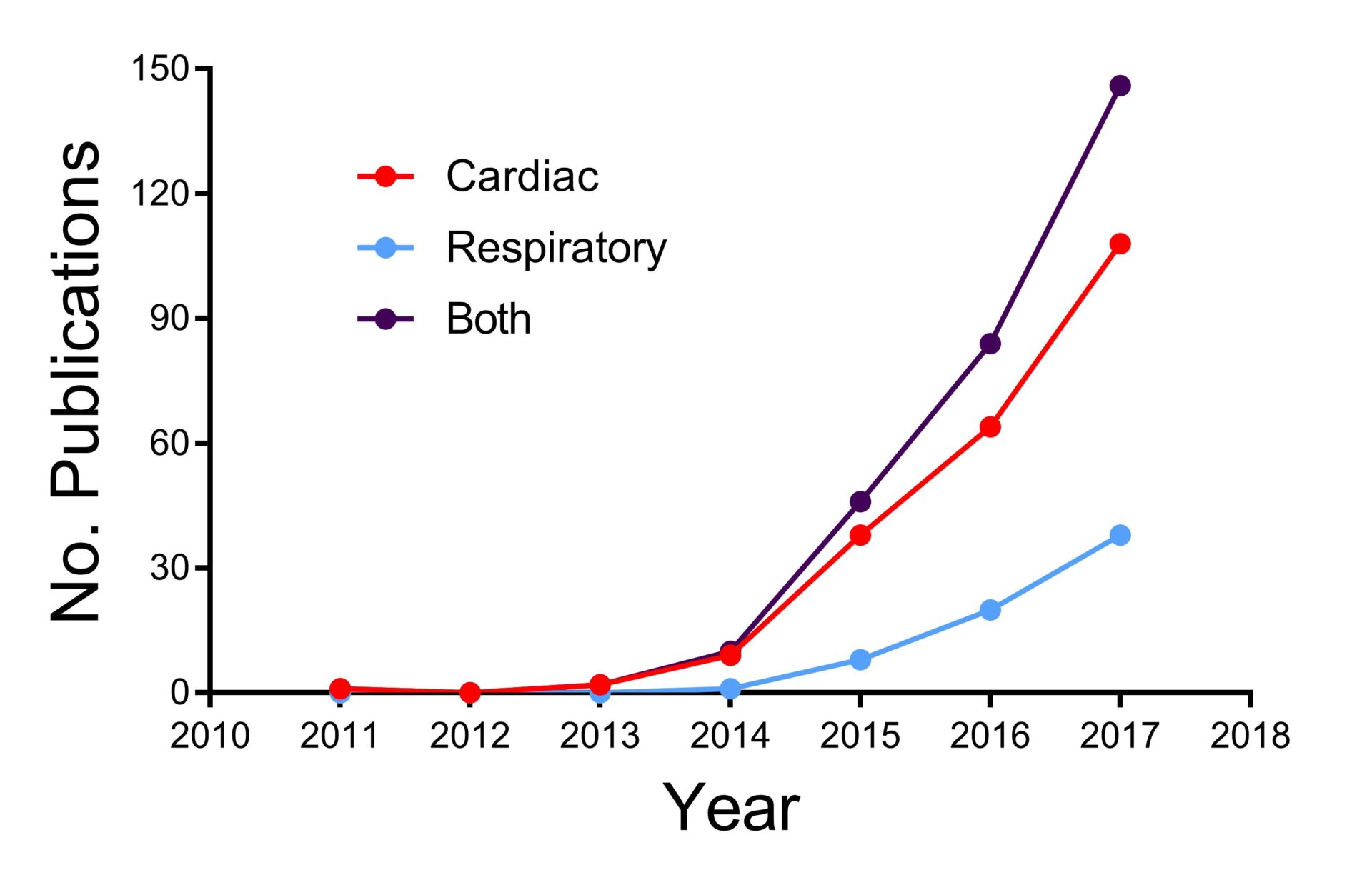
This year will mark the 15th anniversary of the announcement of the sequencing of the human genome. While this important milestone triggered a flurry of studies investigating the links between genomic variation and human disease through genome-wide association studies, in many cases, the physiological mechanisms linking the observed variation with disease remain unclear. A major step forward in this field may come from the UK-based ‘100,000 Genomes Project’ which started in 2012; this ground-breaking enterprise combines medical data with genomic sequences in 70,000 individuals, permitting links to be made between the genome and disease.
Gene editing allows the precise targeting of genes in a range of species and has generated a wealth of information over the last three decades regarding gene function. A fuller introduction to the topic is available in an excellent review by Patrick Harrison (UC Cork) and Stephen Hart
(UCL, London) published very recently in Experimental Physiology (Harrison & Hart, 2018). New gene editing technologies, particularly CRISPR/Cas9, have given recent impetus to research (Fig. 1). In the cardiac and respiratory fields, gene editing is used in rodents and human inducible pluripotent stem cells (hiPSC) to create models and treatment for diseases which are both genetic (long QT syndrome, cystic fibrosis) and multi-factorial (heart failure, chronic obstructive pulmonary disease). However, these approaches are limited by the translational relevance of rodents to humans, and the need to work in the in vitro setting with hiPSCs. This has stimulated the use of genome engineering in species of translational relevance to the human, such as the pig (Yao et al., 2016). These approaches are likely to be valuable in understanding the mechanisms of both common, multifactorial diseases and rare, inherited diseases. To those of us based in the UK, it is encouraging that the British Heart Foundation, a major supporter of cardiovascular research, has emphasised the importance of targeting both common and rare diseases in their research strategy.
As researchers, we may worry what the future holds in terms of funding and our personal financial security, particularly in the UK with uncertainties over Brexit and university pensions. However, in terms of available technologies, there has never been a more exciting time to be a cardiac or respiratory physiologist.
References
Harrison C. (2018). Nanoscopy and cardiovascular physiology. Physiol News 110. doi:
Harrison PT & Hart S. (2018). A beginner’s guide to gene editing. Exp Physiol in press. doi: 10.1113/EP086047
Jayasinghe I, Clowsley AH, Lin R, Lutz T, Harrison C, Green E, Baddeley D, Di Michele L & Soeller C. (2018). True Molecular Scale Visualization of Variable Clustering Properties of Ryanodine Receptors. Cell Rep 22, 557-567. doi: https://doi.org/10.1016/j.celrep.2017.12.045
Patel V. (2017). Deaths registered in England and Wales (series DR): 2016. Statistics OfN, London. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/bulletins/deathsregisteredinenglandandwalesseriesdr/2016
Pinali C, Malik N, Davenport JB, Allan LJ, Murfitt L, Iqbal MM, Boyett MR, Wright EJ, Walker R, Zhang Y, Dobryznski H, Holt CM & Kitmitto A. (2017). Post‐Myocardial Infarction T‐tubules Form Enlarged Branched Structures With Dysregulation of Junctophilin‐2 and Bridging Integrator 1 (BIN‐1). J Am Heart Assoc 6. doi: 10.1161/jaha.116.004834
Yao J, Huang J & Zhao J. (2016). Genome editing revolutionize the creation of genetically modified pigs for modeling human diseases. Hum Genet 135, 1093-1105. doi: 10.1007/s00439-016-1710-6
