
Physiology News Magazine
Getting insight into the work of tendons
Oksana Kostyuk and Robert Brown describe how a novel fibre-optic spectroscopic technique based on light-scattering can provide structural information on tendon changes
Features
Getting insight into the work of tendons
Oksana Kostyuk and Robert Brown describe how a novel fibre-optic spectroscopic technique based on light-scattering can provide structural information on tendon changes
Features
Oksana Kostyuk & Robert Brown
Tissue Repair & Engineering Centre, Institute of Orthopaedics, Royal Free and University College Medical School London, UK
https://doi.org/10.36866/pn.55.28

It is amazing how our quest for knowledge goes in circles. People have always used light reflected by skin for diagnostics. Remember those typical questions: ‘you look a bit pale today, are you all right?’ Or: ‘what is that rash, have you got an allergy?’ Now science is trying to make use of this ancient knowledge in a more systematic and quantitative way. In our centre we use elastic scattering spectroscopy (ESS). Its basic principle is not as dramatic as it sounds: non-harmful white light is flashed onto the surface of the tissue via an optical fibre and the light that comes back from the tissue is collected by a second fibre for analysis. This ‘backscattered’ light contains a wealth of information about the composition and structure of the tissue. A major challenge, however, is to understand just what this overwhelming amount of spectral data means and to ‘dig up’ something useful for diagnostics, for instance to spot early stage skin cancer (Wallace et al. 2000). Another example would be to detect early degeneration of the Achilles’ tendon, for example, after injuries in footballers.
Tendons are the connective tissues which transmit mechanical forces from muscles to bones. To fulfil this function, a rope-like, tough, fibrous structure has evolved, formed by the well-aligned fibrils of collagen, the most abundant body protein. Such a well-organised structural arrangement is characteristic of a healthy tendon, but it becomes less organised as the tendon degenerates, ages or is injured. Loss of this tight-packed organised structure means that the tendon is weaker, less efficient in transmitting forces and prone to break suddenly. Detecting such changes early, predicting an impending rupture, or following the repair after injury would be equally valuable for humans and, for instance, racehorses. At the moment there is no way to measure structural changes in living tendons non-destructively and certainly not without a hospital and a million pound MRI scanner. Both MRI and ultrasound can provide images showing the size and swelling of the tendon, but such qualitative information needs skilled interpretation and remains largely subjective. In addition, the changes detected with these techniques are probably characteristic of rather later stages of the tendon degeneration.
In our recent paper (Kostyuk et al. 2004) we reported how an ESS-based technique was used to non-destructively study structural changes during in vitro loading of the horse superficial digital flexor tendon, analogues to the human Achilles’ tendon. We used the phenomenon of the backscatter anisotropy (unequal distribution) found in tissues formed by aligned fibres like muscle (Marquez et al. 1998) or with partial alignment of structural fibres such as skin (Nickel et al. 2000). When light was delivered to the surface of a normal tendon sample, an elongated, elliptical ‘aura’ of light distribution in the tissue was apparent (Fig. 1). Much more backscatter was detected when the probe was positioned parallel to, rather than perpendicular to, the tendon fibres (the probe was held perpendicular to the tendon surface and rotated to achieve different detection angles). To characterise this backscatter anisotropy we calculated an Anisotropy Factor (AFλ), as a ratio of maximum to minimum intensities of backscatter (at any given wavelength, λ). Where the amount of light travelling in different directions is the same (circle-like ‘aura’), this ratio is one, but it increases rapidly as the backscattered ‘aura’ becomes elliptical. In relaxed tendons the AF500 was around 7, but it increased up to 18.5-fold when tendons were stretched in a material testing machine.
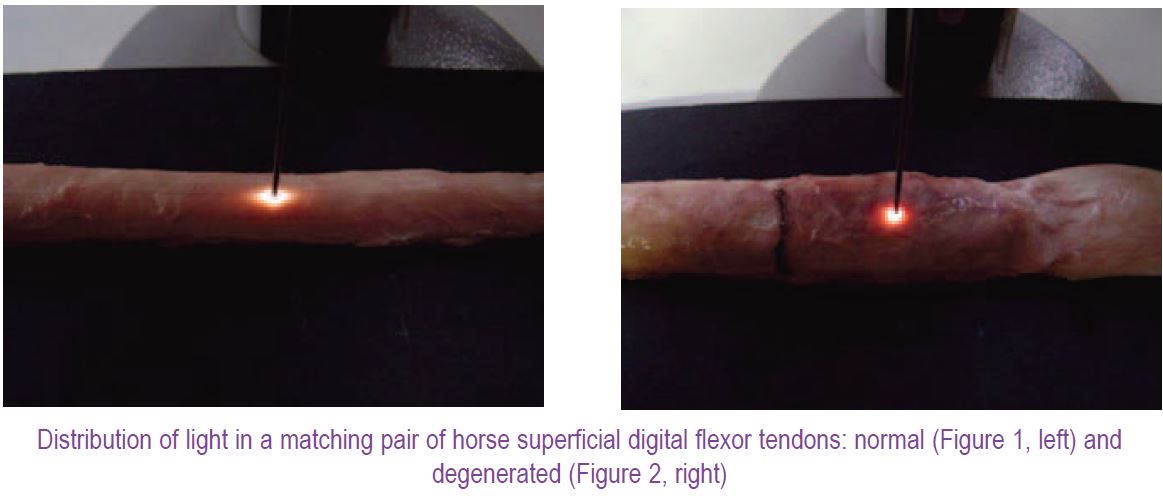
Encouraged, we applied ESS to study tendon pathology, investigating matched pairs of horse tendons, one normal and the other clearly degenerated with a pronounced lesion. This pathological change in the tendon structure produced near circular ‘aura’ of backscatter (Fig. 2). More interestingly, however, even far away from the lesion region of the tendon, the backscatter ‘aura’ was still rounder than the elliptical one of a normal tendon. The AF500 confirmed this impression: it had a value of about 8 for the normal control tendon (similar to the previous study), but in case of the degenerated tendon it was equal to only about 1.2 in the area of the visible lesion and about 2.5 when measured 10 cm away from the lesion. We are now carrying out ultrastructural investigation of these tendons to complement the ESS findings.
Thus, this non-destructive real-time fibre-optical technique can provide structural information on changes in tendons under load or due to pathology. It can be used to study in situ physiological structural changes in tendons and ligaments during use (Morgan et al. in preparation). It has also proved to be an effective means for monitoring development of the collagen fibril alignment as tissue-engineered constructs matured in culture (Kostyuk & Brown, in press). It seems beyond doubt that ESS-based techniques have huge clinical potential as diagnostic and monitoring tools in medicine and tissue engineering, particularly as ESS seems to be able to ‘see’ early structural changes in the tissues. Recent advances in fibre-optic technology make it possible to design really thin optical probes that could be applied via an endo/arthroscope or even via a surgical needle. Together with a relatively cheap basic spectroscopic set-up and a simple data analysis algorithm, this technique may represent a major research and diagnostic advance.
Acknowledgements
The authors are grateful to Helen Birch for providing the horse tendon samples and to Mary Morgan for her help with the imaging of the tendons.
References
Kostyuk O & Brown RA (in press). Novel spectroscopic technique for in situ monitoring of collagen fibril alignment in gels. Biophys J.
Kostyuk O, Birch HL, Mudera V & Brown RA (2004). Structural changes in loaded tendons can be monitored by a novel spectroscopic technique. J Physiol 554, 791-801.
Marquez G, Wang LV, Lin SP, Schwartz JA & Thomsen SL (1998). Anisotropy in the absorption ans scattering spectra of chicken breast tissue. Appl Optics-OT 37(4), 798-804.
Nickel S, Hermann M, Essenpreis M, Farrell TJ, Krämer U & Patterson MS (2000). Anisotropy of light propagation in human skin.
Wallace VP, Crawford DC, Mortimer PS, Ott RJ & Bamber JC (2000). Spectrophotometric assessment of pigmented skin lesions: methods and feature selection for evaluation of diagnostic performance. Phys Med Biol 45(3), 735-751.
