
Physiology News Magazine
Glucocorticoid regulation of blood brain barrier permeability
Features
Glucocorticoid regulation of blood brain barrier permeability
Features
Carola Förster
Institute of Anatomy and Cell Biology, University of Würzburg, Koellikerstrasse 6, D-97070 Würzburg, Germany
https://doi.org/10.36866/pn.61.34

Homeostasis of the central nervous system (CNS) microenvironment is essential for its normal function. In the early l900s researchers found the first evidence that the brain had a specialized barrier that protected its cells. Dyes injected into the body’s blood supply would stain the tissues of most organs – but not the brain. It is now known that a ‘blood-brain barrier’ (BBB) keeps many substances out of the brain. The walls of the vessels (endothelial cells) that carry blood into the brain form the barrier.
The main structures responsible for this barrier property are the tight junctions (TJ). Tight junctions (occluding junctions) are cell-cell junctions that seal adjacent endothelial cells of the BBB together, preventing the passage of most ions and hydrophilic macromolecules from one side of the endothelial sheet to the other. Cadherinbased adherens junctions in close proximity are important for mechanical stabilisation of the TJ (Fig. 1). TJ are strongly developed in endothelial cells of the blood brain barrier (BBB) but only moderately formed between endothelial cells of the peripheral vasculature: leaky blood vessels in the body allow many molecules to cross through to tissue, but the tight construction of the vessels in the CNS guards against brain entry. BBBforming brain capillary endothelial cells (BCECs) express the TJ proteins occludin and claudin-5 (Rubin & Staddon, 1999).

Many diseases of the CNS, such as stroke, brain tumours, traumatic injury or multiple sclerosis, are typically accompanied by dysfunction of the BBB. Therapeutic strategies for several of these diseases include treatment with glucocorticoids (GC) (Engelhardt, 2000) but the molecular basis of how GC regulate BBB permeability is not well understood. GCs are routinely administered for the management of the effects of stroke and brain edema. Clinical reports further describe the barrier closing effects of GCs on MRI gadolinium enhancement in acute demyelinating lesions, a marker of active blood-brain barrier damage secondary to an inflammatory process (Burnham et al. 1991). Complementary to this, plenty of data have been accumulated on the barrier tightening effects of GCs in in vitro systems of the BBB (for review see Rubin & Staddon, 1999).
It is known that GC effects are mediated by a member of the superfamily of nuclear hormone receptors, the glucocorticoid receptor (GR). These proteins influence gene expression via the activation or repression, respectively, of a given gene (Beato, 1989). Thus, the GR acts as a transcriptional regulator. The GR response elements are DNA sequences of the promoter (transcription regulatory element) of a given target gene that serve as binding sites for the GC-activated GR. The identification of GR target genes, and thus elucidation of the underlying molecular mechanisms leading to GC-mediated BBB improvement, was hampered in the past by the lack of a genetically defined in vitro system, which would be easy and reproduceable to handle and allow for genetic manipulation. Research thus far has been largely limited to primary cultured BCECs from rat, bovine or porcine origin or immortalised rat brain endothelial cells and thus did not allow for dissection of molecular events. Consequently, no direct target genes for the GR could be identified, so that only indirect mechanisms influencing gene expression have been discussed in detail (Engelhardt, 2000).
Recently, the establishment of a murine immortalised BCEC cell culture system (cEND) which is responsive to GC has allowed the identification of target genes of the GR in BCECs. Administration of GCs at physiological concentrations led to an elevated expression of occludin protein and lower permeability in cEND BCECs. Transactivation of the human occludin promoter by the GR in the presence of GCs could be demonstrated in vitro using a luciferase-coupled promoterreporter construct. These observations led to our understanding that GCs increase barrier properties of BCECs by inducing enhanced expression of the TJ transmembrane component occludin via binding of the activated GR to putative GC responsive elements (GRE) in the occludin promoter (Fig. 2) (Förster et al., 2005).
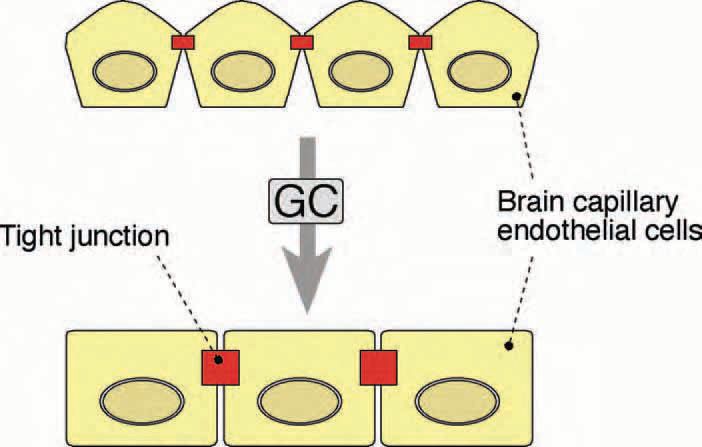
Long-term treatment with high levels of GCs causes a range of severe side effects, such as weight gain concomitant with fat redistribution (Cushing syndrome), GC-induced hypertension, hyperglycemia (diabetes mellitus) and osteoporosis (Kimberly, 1991). Further dissection of the molecular events regulating gene transcription at the BBB should therefore be beneficial for the future development of target cell-specific GR ligands, ultimately as a therapeutic strategy.
References
Beato M (1989). Gene regulation by steroid hormones. Cell 56, 335344.
Burnham JA, Wright RR, Dreisbach J & Murray RS (1991). The effect of high-dose steroids on MRI gadolinium enhancement in acute demyelinating lesions. Neurology 41, 1349-1354.
Engelhardt B (2000). Role of glucocorticoids on T cell recruitment across the blood-brain barrier. Z Rheumatol 59 Suppl 2, II/18-21.
Förster C, Silwedel C, Golenhofen N, Burek M, Kietz S, Mankertz J & Drenckhahn D (2005). Occludin as direct target for glucocorticoidinduced improvement of blood brain-barrier properties in a murine in vitro system. J Physiol 565(Pt 2), 475-486.
Kimberly RP (1991). Mechanisms of action, dosage schedules, and side effects of steroid therapy. Curr Opin Rheumatol 3, 373-379.
Rubin LL & Staddon JM (1999). The cell biology of the blood-brain barrier. Annu Rev Neurosci 22, 11-28.
