Physiology News Magazine
Going with the flow: just say NO to oxygen radicals
Endothelial cells are permanently exposed to shear stress by the flowing blood. Shear stress increases the endothelial formation of nitric oxide. But what happens to oxygen radicals during flow? Henning Morawietz and colleagues analyzed the flow-dependent regulation of the NADPH oxidase as a major source of endothelial oxidative stress
Features
Going with the flow: just say NO to oxygen radicals
Endothelial cells are permanently exposed to shear stress by the flowing blood. Shear stress increases the endothelial formation of nitric oxide. But what happens to oxygen radicals during flow? Henning Morawietz and colleagues analyzed the flow-dependent regulation of the NADPH oxidase as a major source of endothelial oxidative stress
Features
Henning Morawietz
Department of Vascular Endothelium and Microcirculation, Medical Faculty Carl Gustav Carus, University of Technology Dresden, Dresden, Germany.
https://doi.org/10.36866/pn.66.30

We all know the good advice of our physician to exercise on a regular basis. From the physiological point of view, a major beneficial effect of this everyday challenge could be an increase in blood flow. As the inner layer of our blood vessels, the endothelial cells are permanently exposed to shear stress throughout their lifetime. A well-known beneficial effect of increased shear stress is the augmented endothelial formation of nitric oxide (NO). NO does not only mediate endotheliumdependent vasodilation, but also antiinflammatory and anti-thrombotic processes (Landmesser et al. 2004). Therefore, formation of NO by the endothelial NO synthase (eNOS) is a critical determinant of endothelial function. The NO availability can be limited by enhanced formation of reactive oxygen species like superoxide anions (·O2-) (Bachschmid et al. 2005). NO and ·O2- can form peroxynitrite in a very rapid reaction, thus reducing the available NO. An increased formation of reactive oxygen species has been considered as a major determinant of endothelial dysfunction (Harrison et al. 2003). Another detrimental effect of vascular oxidative stress is the increased oxidative modification of low-density lipoprotein thus promoting foam cell formation and atherosclerosis.

While several studies have addressed the impact of shear stress on NO formation, much less is known about the impact of shear stress on endothelial formation of reactive oxygen species. Our lab established a cone-and-plate viscometer as an experimental model to simulate different levels of shear stress on cultured endothelial cells more than 10 years ago (Fig. 1). Because the NADPH oxidase has been identified as a major source of reactive oxygen species like ·O2- in human endothelial cells (Rueckschloss et al. 2003), in a recent study we analyzed the impact of laminar shear stress on ·O2- formation and NAD(P)H oxidase subunit expression in human endothelial cells (Duerrschmidt et al. 2006). Short-term application of shear stress transiently induced ·O2- formation in human endothelial cells. This was inhibited by the NAD(P)H oxidase-specific inhibitor gp91ds-tat, but NAD(P)H oxidase subunit expression was unchanged. In contrast, long-term arterial laminar shear stress downregulated ·O2formation, mRNA and protein expression of NAD(P)H oxidase subunits Nox2/gp91phox and p47phox. In parallel, endothelial NO formation and eNOS, but not Cu/Zn superoxide dismutase expression was increased. Interestingly, downregulation of ·O2formation, gp91phox and p47phox expression by long-term laminar shear stress was blocked by eNOS inhibition. Furthermore, an NO donor downregulates ·O2 formation, gp91phox and p47phox expression in static endothelial cells. Our data suggest a transient activation of ·O2- formation by short-term shear stress, followed by a downregulation of endothelial NAD(P)H oxidase in response to longterm laminar shear stress. NO-mediated downregulation by shear stress preferentially affects the gp91phox/p47phox-containing NAD(P)H oxidase complex. This novel mechanism might be involved in the shear stress-dependent regulation of the endothelial NO/·O2- balance (Fig. 2). An increased NO and decreased ·O2formation could also contribute to the vasoprotective potential of physiological levels of laminar shear stress. This would further support an intact and healthy endothelial function.
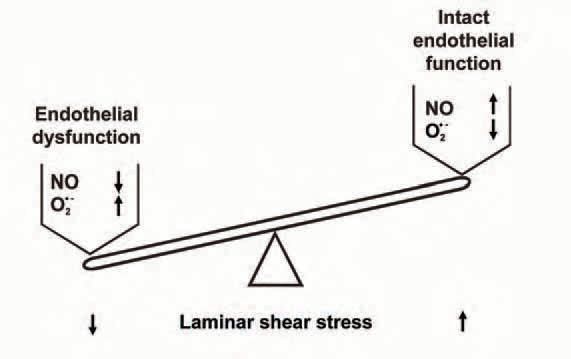
How can this be translated into cardiovascular physiology and pathophysiology? Short-term and longterm endothelial NO formation by shear stress can involve different mechanisms. While the first phase mainly represents a functional activation of eNOS, the second phase is accompanied by an upregulation of eNOS expression (Fleming & Busse, 2003). Similarly, short-term shear stress seems to activate NADPH oxidase activity. In contrast, long-term application of laminar shear stress downregulates ·O2- formation and NADPH oxidase subunit expression. This makes physiological sense because both processes improve the vascular NO availability in response to increased laminar flow. Interestingly, NO even seems to be involved in the downregulation of oxidative stress by long-term laminar flow. This is not just a simple scavenging of ·O2- by NO, because transcriptional downregulation of NADPH oxidase subunit expression by long-term laminar shear stress involves an NO-dependent pathway as well. Which NO-dependent transcription factors are involved in this process is currently not known.
Coming back to the initial advice of our physician, should we also exercise to balance our NO/·O2 − ratio? The answer is definitely yes. Experimental studies in flow-adapted coronary arterioles and aorta support this concept. Increased blood flow in mice subjected to − voluntary training reduces vascular ·O2 release and NADPH oxidase subunit expression as well (Laufs et al. 2005). Finally, chronic exercise training of patients with coronary artery disease increases flow, decreases generation of reactive oxygen species and expression of gp91phox in internal mammary arteries, and improves endothelial function (Adams et al. 2005).
The bad news for all couch potatoes: the beneficial effects of exercise are only transient. We have to move on.
Acknowledgements
This study was supported by the German Federal Ministry of Education and Research (BMBF) program NBL3 of the University of Technology Dresden.
References
Adams V, Linke A, Krankel N, Erbs S, Gielen S, Mobius-Winkler S, Gummert JF, Mohr FW, Schuler G & Hambrecht R (2005). Impact of regular physical activity on the NAD(P)H oxidase and angiotensin receptor system in patients with coronary artery disease. Circulation 111, 555-562.
Bachschmid M, Schildknecht S & Ullrich V (2005). Redox regulation of vascular prostanoid synthesis by the nitric oxide-superoxide system. Biochem Biophys Res Comm 338, 536-542.
Duerrschmidt N, Stielow C, Muller G, Pagano PJ & Morawietz H (2006). NO-mediated regulation of NAD(P)H oxidase by laminar shear stress in human endothelial cells. J Physiol 576, 557-567.
Fleming I & Busse R (2003). Molecular mechanisms involved in the regulation of the endothelial nitric oxide synthase. Am J Physiol Regul Integr Comp Physiol 284, R1-12.
Harrison D, Griendling KK, Landmesser U, Hornig B & Drexler H (2003). Role of oxidative stress in atherosclerosis. Am J Cardiol 91, 7A-11A.
Landmesser U, Hornig B & Drexler H (2004). Endothelial function: a critical determinant in atherosclerosis? Circulation 109, II27-33.
Laufs U, Wassmann S, Czech T, Munzel T, Eisenhauer M, Bohm M & Nickenig G (2005). Physical inactivity increases oxidative stress, endothelial dysfunction, and atherosclerosis. Arterioscler Thromb Vasc Biol 25, 809-814.
Rueckschloss U, Duerrschmidt N & Morawietz H (2003). NADPH oxidase in endothelial cells: impact on atherosclerosis. Antioxid Redox Signal 5, 171-180.