Physiology News Magazine
Heart rate regulation during exercise
Exercise-mediated increases in heart rate are elicited by a complex interaction of multiple neural control mechanisms
Features
Heart rate regulation during exercise
Exercise-mediated increases in heart rate are elicited by a complex interaction of multiple neural control mechanisms
Features
James P. Fisher (1), Niels H. Secher (2) and Paul J. Fadel (3)
1: School of Sport & Exercise Sciences, University of Birmingham, Edgbaston, Birmingham B15 2TT, UK
2: Copenhagen Muscle Research Center, Department of Anaesthesia, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark
3: Department of Medical Pharmacology and Physiology, Dalton Cardiovascular Research Center, University of Missouri, Columbia, MO, USA
https://doi.org/10.36866/pn.81.41
Exercise-mediated increases in heart rate are elicited by a complex interaction of multiple neural control mechanisms. We have demonstrated that the activation of metabolically sensitive receptors in skeletal muscles (muscle metaboreflex) increases cardiac sympathetic nerve activity in humans, an effect that can be masked by elevations in parasympathetic tone. Thus, the muscle metaboreflex contributes importantly to the regulation of the heart during exercise. These findings have implications for disease conditions associated with low cardiac parasympathetic tone and exaggerated feedback from the exercising muscles, such as chronic heart failure.

Profound alterations in cardio-vascular regulation must occur in order to sustain exercise for more than a few moments. To meet the increased metabolic demand of the active muscles, local blood flow must increase. Consequently, cardiac output increases and blood flow is redirected to the contracting muscles by vasoconstriction in regions such as renal and splanchnic vascular beds. Both the sympathetic and parasympathetic branches of the autonomic nervous system are important in mediating this co-ordinated response and the elucidation of the underlying regulatory mechanisms has engaged researchers for a century. It is now established that the cardiovascular responses to exercise result from the activation and interaction of both central and peripheral neural mechanisms. Feed-forward signals from the brain (e.g. insular cortex), known as central command, arise in parallel with descending motor drive to the exercising muscles and converge on the cardiovascular areas of the brain (e.g. nucleus tractus soltarii).
Concomitant feedback from the active muscles via small group III and IV afferent nerves provides feedback to these cardiovascular areas in response to both mechanical and metabolic stimulation. As a consequence, the arterial baroreflex is reset to the prevailing heart rate and blood pressure established during exercise and this plays an important role in the neural regulation of the cardiovascular system during physical activity.
The dissection of the discrete contribution made by each of the neural control mechanisms implicated in the cardiovascular response to exercise is challenging, particularly in human studies where invasive methods are limited. However, an array of ingenious experimental approaches (e.g. neuro muscular blockade, deep brain stimulation, tendon vibration and inflatable trousers) along with deductive reasoning has brought insight to this research area.
One approach that is commonly used in humans to investigate the cardiovascular effects of activating metabolically sensitive skeletal muscle afferents (muscle metaboreflex) involves the occlusion of the circulation to the exercising muscles just prior to the end of exercise and keeping it in place for a period of exercise recovery. This ‘post-exercise ischaemia’ traps the metabolites produced during exercise within the muscles after the contraction and effectively isolates the muscle metaboreflex from the exercise-induced activation of central command and mechanically sensitive muscle afferents. The circulatory occlusion can be performed simply by inflation of a cuff around the limb, proximal to the exercising muscles, as first described by Alam & Smirk (1937). Alarmingly these pioneering investigators also noted that ‘a forearm may be devascularied and the circulation arrested by plunging it into a bath of mercury depth of about 12 cm. above the elbow’, but thankfully this approach has not been adopted!

Intriguingly, heart rate consistently falls to baseline values during post-exercise ischaemia while exercise-induced increases in blood pressure and vasoconstrictor sympathetic nerve activity remain elevated. This has led to the notion that the muscle metaboreflex does not influence heart rate, but raises blood pressure via sympathetically mediated peripheral vasoconstriction (Rowell & O’Leary, 1990). An alternative explanation is that an overwhelming effect of parasympathetic reactivation slows the heart during post-exercise ischaemia, and masks the influence of sympathetic nerve activity on the heart (Fig. 1). This may occur for two reasons. First, the inhibitory effects of central command and mechanically sensitive skeletal muscle afferents on parasympathetic activity are lost in the transition from exercise to post-exercise ischaemia. Second, the elevation in blood pressure during post-exercise ischaemia could stimulate the arterial baroreceptors and reflexively increase parasympathetic tone. As such, this parasympathetic reactivation could obscure any metaboreflex-mediated increase in cardiac sympathetic nerve activity which would otherwise accelerate heart rate (O’Leary, 1993). If this were the case, then the elimination of cardiac parasympathetic tone during post-exercise ischaemia should reveal an increase in heart rate, as a muscle metaboreflex-mediated increase in cardiac sympathetic nerve activity would be unmasked.
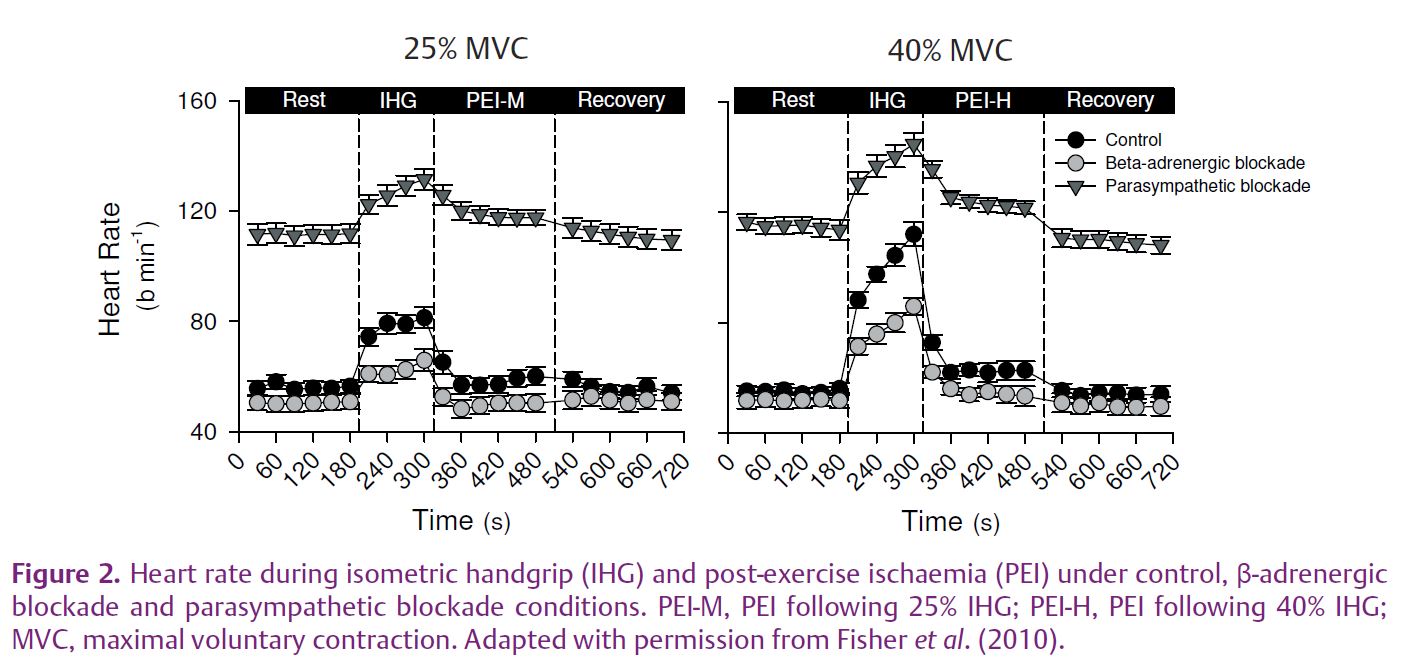
To investigate the potential influence of sympathetic nerve activity on heart rate during post-exercise ischaemia we used a pharmacological approach previously performed in exercising dogs (O’Leary, 1993). The cardiovascular responses to two intensities of muscle metaboreflex activation were compared during post-exercise ischaemia under control conditions, and after parasympathetic and β-adrenergic blockade (Fisher et al. 2010). As expected, blood pressure was increased from rest during moderate metaboreflex activation and further elevated during high-intensity metaboreflex activation in all conditions. We observed that during moderate-intensity metaboreflex activation under control (no drug) conditions, heart rate was negligibly elevated from rest (+3±2 beats min–1); however, when this was repeated following parasympathetic blockade (using the muscarinic blocker glycopyrrolate) an increase in heart rate was observed (+8±2 beats min-–1; Fig. 2). Interestingly, when high-intensity metaboreflex activation was performed an elevation in heart rate was noted, which was attenuated with β-adrenergic blockade but was unchanged with parasympathetic blockade. Collectively, these findings suggest that the muscle metaboreflex increases cardiac sympathetic nerve activity during post-exercise ischaemia in humans; however, it requires a robust muscle metaboreflex activation to offset the influence of cardiac parasympathetic reactivation on heart rate.

We also observed that the rate at which heart rate recovered from the end of exercise during post-exercise ischaemia was slower with parasympathetic blockade, compared with the control or β-adrenergic blockade conditions (Fig. 3). We presume that this sluggish heart rate recovery is due to gradual withdrawal of cardiac sympathetic nerve activity following the rapid loss of inputs from central command and muscle mechanoreceptors during sustained muscle metaboreflex activation. These data are important because a delayed recovery in heart rate following exercise is a powerful independent predictor of mortality even in low risk patient populations (Cole et al. 1999), an effect that is linked to the reactivation of parasympathetic activity (Imai et al. 1994). Our findings broadly support this concept, and further suggest that in the absence of parasympathetic reactivation, increased cardiac sympathetic nerve activity may also contribute to a delayed post-exercise recovery of heart rate. These data may be clinically relevant for disease conditions associated with altered skeletal muscle afferent sensitivity and low cardiac parasympathetic tone, such as chronic heart failure.
In summary, these findings suggest that isolated muscle metaboreflex activation increases cardiac sympathetic nerve activity during post-exercise ischaemia in humans, but to have an effect on heart rate, robust muscle metaboreflex activation is needed to offset cardiac parasympathetic reactivation.
References
Alam M & Smirk FH (1937). Observations in man upon a blood pressure raising reflex arising from the voluntary muscles. J Physiol 89, 372–383.
Cole CR, Blackstone EH, Pashkow FJ, Snader CE & Lauer MS (1999). Heart-rate recovery immediately after exercise as a predictor of mortality. N Engl J Med 341, 1351–1357.
Fisher JP, Seifert T, Hartwich D, Young CN, Secher NH & Fadel PJ (2010). Autonomic control of heart rate by metabolically sensitive skeletal muscle afferents in humans. J Physiol 588, 1117–1127. http://jp.physoc.org/content/588/7/1117.long
Imai K, Sato H, Hori M, Kusuoka H, Ozaki H, Yokoyama H et al. (1994). Vagally mediated heart rate recovery after exercise is accelerated in athletes but blunted in patients with chronic heart failure. J Am Coll Cardiol 24, 1529–1535.
O’Leary DS (1993). Autonomic mechanisms of muscle metaboreflex control of heart rate. J Appl Physiol 74, 1748–1754.
Rowell LB & O’Leary DS (1990). Reflex control of the circulation during exercise: chemoreflexes and mechanoreflexes. J Appl Physiol 69, 407–418.