
Physiology News Magazine
Hepatic insulin resistance is induced by high-fat overfeeding in healthy men
Diets high in fat and calories are associated with increased risk of obesity and type 2 diabetes; however, the contribution of metabolic defects to the development type 2 diabetes is controversial
Features
Hepatic insulin resistance is induced by high-fat overfeeding in healthy men
Diets high in fat and calories are associated with increased risk of obesity and type 2 diabetes; however, the contribution of metabolic defects to the development type 2 diabetes is controversial
Features
Charlotte Brøns
Steno Diabetes Center, Niels Steensens Vej 1, 2820 Gentofte, Denmark
https://doi.org/10.36866/pn.76.12

Recent findings have shown that high-fat overfeeding for 5 days results in insulin resistance of the liver but not of the muscle tissue and in increased insulin secretion. Since increased insulin secretion precedes the development of muscle insulin resistance after overfeeding, it is suggestive of a role for insulin in the development of muscle insulin resistance and obesity. Taken together, this underscores the significant adverse effects of fat overfeeding in healthy humans, even during short-term exposures equivalent to commonly occurring feast periods in most societies.
High-fat diet and development of type 2 diabetes
High-fat calorie-rich diets along with physical inactivity have made type 2 diabetes a worldwide epidemic. Although many metabolically active organs may be involved, the development of type 2 diabetes is characterized by three major defects – insulin resistance of the muscle tissue, elevated hepatic glucose production and impaired insulin secretion. However, the relative contribution of muscle and hepatic insulin resistance versus defective insulin secretion on development of hyperglycaemia is controversial, and may depend on the dominant underlying aetiology of this multi-factorial disease.
Altered lipid metabolism plays an important role in the pathogenesis of insulin resistance. Accumulation of excess fat as intramyocellular lipid (IMCL) in muscle tissue has been reported to be associated with reduced insulin sensitivity, and it has been shown that mitochondrial function and expression of genes involved in oxidative phosphorylation (OXPHOS) including their key co-transcriptional factor peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) are commonly decreased in elderly and obese insulin-resistant and type 2 diabetic subjects (Patti et al. 2003; Petersen et al. 2004). Dysregulation of intramyocellular lipid (IMCL) metabolism in insulin resistance and type 2 diabetes may be linked with defective OXPHOS, and both short- and long-term fat exposure may play a key role in the development of impaired mitochondrial OXPHOS.
Previous studies have used less-physiological fat exposure experiments using intravenous lipid infusions to induce supraphysiological high levels of plasma free fatty acids (FFA) as a model to study the metabolic effects of high fat exposure, and thereby to mimic the state of overt type 2 diabetes commonly characterized by elevated FFA levels. Other studies have used varying duration of either different types of overfeeding or diets containing high amounts of fat. These studies have mainly included rodents, obese human subjects and/or human subjects with a family history of diabetes, using relatively small numbers. However, the literature is relatively limited with regards to studies of the acute effects of a physiological diet high in both fat and calories on metabolic mechanisms relevant to the pathophysiology of type 2 diabetes in healthy subjects.
Impact of short-term high-fat overfeeding on hepatic versus muscle insulin resistance
We recently reported that intake of a high-fat diet (60%) with 50% extra calories for 5 days in healthy young men resulted in an approximately 25% increase of fasting hepatic glucose production (Fig. 1A), in spite of an elevated fasting insulin level (Brøns et al. 2009). As a consequence of the increased hepatic glucose production, fasting glucose levels were increased by 0.5 mmol l–1 after overfeeding, and although within the normal range, elevated fasting glucose does in itself constitute an independent risk factor for developing type 2 diabetes. The fact that only 5 days of high-fat overfeeding increases fasting glucose levels due to hepatic insulin resistance underscores the significant deleterious effects of fat overfeeding per se in healthy humans even during short-term exposures, equivalent to commonly occurring feast periods in most societies. When calculating the hepatic insulin resistance index, the finding of an elevated hepatic glucose production after overfeeding was shown to be due to hepatic insulin resistance (Fig. 1B). In addition, overfeeding resulted in a significant increase of the liver enzyme aspartate aminotransferase (ASAT), an indication of a stressed liver accumulating fat. Indeed, hepatic steatosis in human subjects is increasingly recognized as a key feature in the metabolic syndrome associated with development of hepatic insulin resistance.
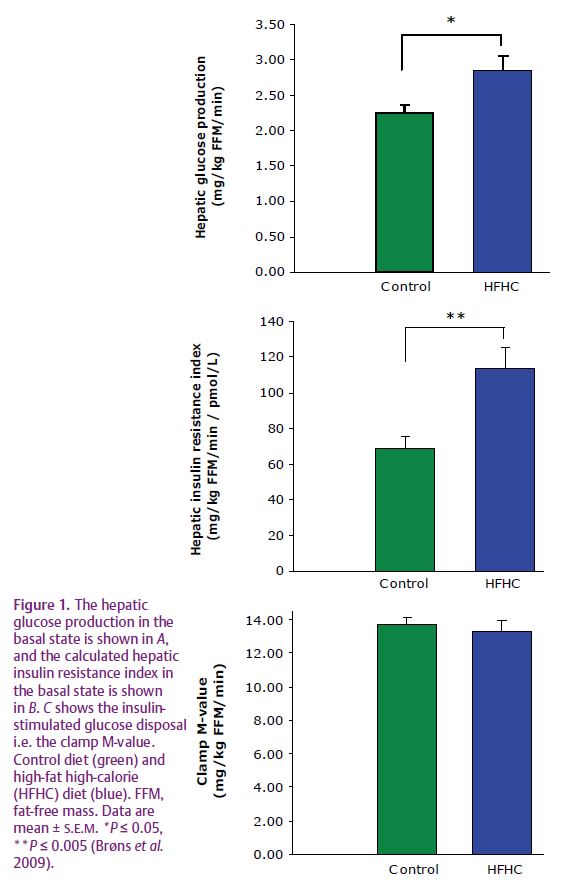
Despite the observed hepatic insulin resistance, whole-body peripheral (muscle) insulin-stimulated glucose uptake was not decreased after fat overfeeding (Fig. 1C). Previous studies of short-term fructose and carbohydrate overfeeding did not have an effect on whole-body insulin sensitivity in lean healthy subjects either, and the finding of hepatic insulin resistance without the presence of muscle insulin resistance after only 5 days of fat overfeeding has not been shown in healthy subjects before. Despite normal whole-body glucose uptake, the insulin-stimulated glycolysis was decreased by approximately 25% by overfeeding, consistent with reports of decreased insulin-stimulated glycolysis preceding overt peripheral insulin resistance during intravenous lipid infusion. However, in vivo mitochondrial function was not affected by high-fat overfeeding when measured by 31P-magnetic resonance spectroscopy before and after energy-depleting exercise in two different muscle groups. Furthermore, no differences in basal or insulin-stimulated expression of PGC-1α and OXPHOS genes in muscle tissue were observed either. The data therefore do not support the current hypothesis that a short-term high-fat diet reduces the expression of nuclear genes encoding mitochondrial transcription factors involved in mitochondrial biogenesis in healthy young men (Sparks et al. 2005).
Assessment of β-cell function in relation to insulin action
Acute intravenous lipid exposure and FFA elevation increases insulin secretion whereas chronic elevation of FFA may cause β-cell dysfunction as a consequence of lipotoxicity. The first-phase insulin response to an intravenous glucose bolus is a sensitive measure of β-cell function. However, assessment of relative β-cell function in humans is challenging because of the complex interplay between insulin secretion, insulin sensitivity and hepatic insulin extraction (Cobelli et al. 2007).
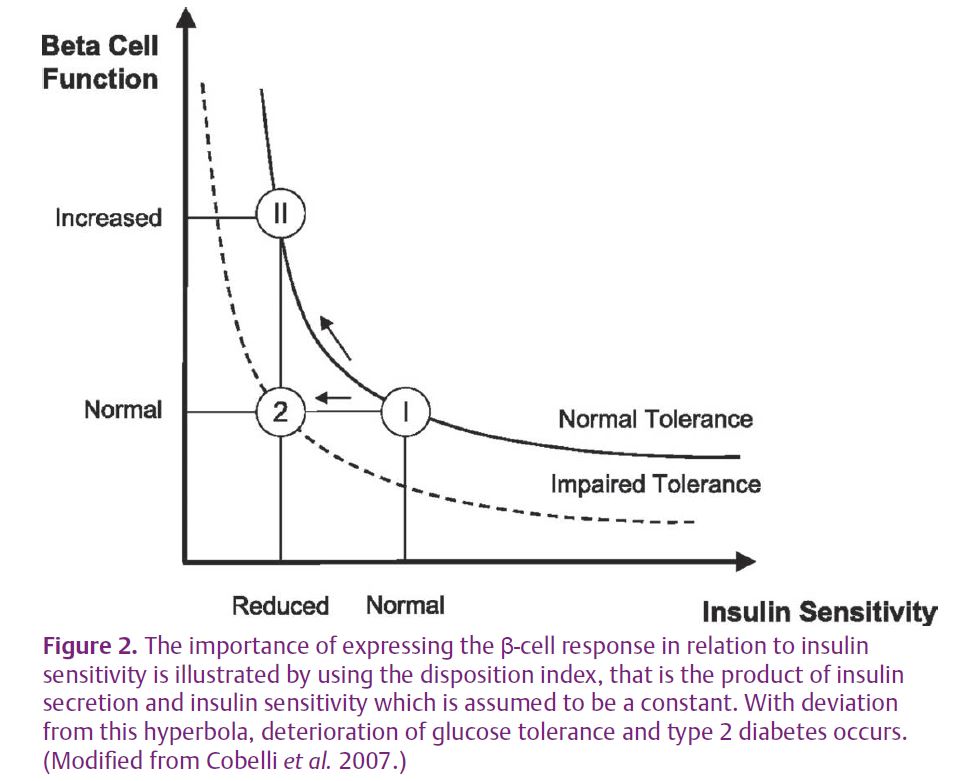
In individuals with normal glucose tolerance, an approximate hyperbolic relation exists between β-cell function and insulin sensitivity, where a reduction in insulin sensitivity is accompanied by a compensatory upregulation of insulin secretion (Fig. 2). This relationship between insulin sensitivity and insulin secretion, commonly known as the disposition index, is determined as the product of the two variables, and is thought to be constant for individuals with the same degree of glucose tolerance. Although the conventional view is that increased insulin secretion is a secondary and compensatory response to insulin resistance (Bergman et al. 2002), data suggest that the inverse scenario with increased insulin secretion preceding and possibly causing insulin resistance may characterize some metabolic states (Le Stunff & Bougnères, 1994). Interestingly, partly due to insufficient knowledge about the molecular feedback mechanisms and signals between β-cell and insulin action mediating the inverse relationship between the two, the extent to which hepatic or muscle insulin action should be used in the calculation of the disposition index, an issue clearly relevant to data showing discordant results of high-fat overfeeding on hepatic versus muscle insulin action, has not even been debated.
We showed that high-fat overfeeding resulted in significantly elevated insulin secretion, and the disposition index was subsequently calculated in two ways: either hepatic or muscle insulin action. When including muscle insulin action in the calculation, a disproportionately elevated insulin secretion in relation to muscle insulin action was observed. However, when including hepatic insulin action, the disposition index was unaltered by high-fat feeding, indicating appropriate compensation of the β-cell. This indicates that the increased insulin secretion could possibly compensate for the hepatic insulin resistance and precede the development of muscle insulin resistance in young men, thereby suggesting that hyperinsulinaemia may be causally related to the development of muscle insulin resistance and obesity after longer periods of high-fat feeding. However, we are not able to determine the extent to which increased insulin secretion may precede the development of hepatic insulin resistance. Taken together, the data therefore indicate that the adjustment for hepatic insulin sensitivity in the calculation of the disposition index provides different information to that of peripheral insulin sensitivity, and helps to improve our understanding of the complexity of in vivo estimation of β-cell function in relation to insulin action.
References
Bergman RN, Finegood DT & Kahn SE (2002). The evolution of β-cell dysfunction and insulin resistance in type 2 diabetes. Eur J Clin Invest 32, 35–45.
Brøns C, Jensen CB, Storgaard H, Hiscock NJ, White A, Appel JS, Jacobsen S, Nilsson E, Larsen CM, Astrup A, Quistorff B, & Vaag A (2009). Impact of short-term high-fat feeding on glucose and insulin metabolism in young healthy men. J Physiol 587, 2387–2397. http://jp.physoc.org/content/587/10/2387. long
Cobelli C, Toffolo GM, Man CD, Campioni M, Denti P, Caumo A, Butler P & Rizza R (2007). Assessment of β-cell function in humans, simultaneously with insulin sensitivity and hepatic extraction, from intravenous and oral glucose tests. Am J Physiol Endocrinol Metab 293, E1–E15.
Le Stunff C & Bougnères P (1994). Early changes in postprandial insulin secretion, not in insulin sensitivity, characterize juvenile obesity. Diabetes 43, 696–702.
Patti ME, Butte AJ, Crunkhorn S, Cusi K, Berria R, Kashyap S et al. (2003). Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: Potential role of PGC1 and NRF1. Proc Natl Acad Sci U S A 100, 8466–8471.
Petersen KF, Dufour S, Befroy D, Garcia R & Shulman GI (2004). Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med 350, 664-671.
Sparks LM, Xie H, Koza RA, Mynatt R, Hulver MW, Bray GA & Smith SR (2005). A high-fat diet coordinately downregulates genes required for mitochondrial oxidative phosphorylation in skeletal muscle. Diabetes 54, 1926–1933.
