
Physiology News Magazine
Hit the endothelial layer of your skeletal muscle microvessels with HIT to prevent impaired glucose tolerance and chronic disease
The human body, and specifically the microcirculatory system, are sensitive to physical activity or the lack thereof. Health authorities tell us repeatedly that obesity, metabolic syndrome, type 2 diabetes and cardiovascular disease threaten the lives of the sedentary, but few of us manage to meet recommended exercise guidelines. Why is exercise so important to the microvasculature and is HIT really an effective short-cut to maintaining good health?
Features
Hit the endothelial layer of your skeletal muscle microvessels with HIT to prevent impaired glucose tolerance and chronic disease
The human body, and specifically the microcirculatory system, are sensitive to physical activity or the lack thereof. Health authorities tell us repeatedly that obesity, metabolic syndrome, type 2 diabetes and cardiovascular disease threaten the lives of the sedentary, but few of us manage to meet recommended exercise guidelines. Why is exercise so important to the microvasculature and is HIT really an effective short-cut to maintaining good health?
Features
Anton Wagenmakers & Matthew Cocks
Liverpool John Moores University, UK
https://doi.org/10.36866/pn.93.22
The microcirculation in human skeletal muscle is highly responsive to increases and decreases in habitual physical activity with substantial changes in capillary density and activity of endothelial enzymes that control muscle perfusion. Endurance trained athletes combine a high skeletal muscle capillary density with a microvasculature that is highly responsive to meal- and exercise-induced vasodilatation. Individuals that adopt a sedentary lifestyle, including the rapidly growing obese and ageing population, progressively lose muscle capillaries, oxidative capacity and mass. These impairments go in parallel with a high chronic disease risk (metabolic syndrome, type 2 diabetes and cardiovascular disease). This article explains the state of knowledge of the underlying mechanisms and comes to the conclusion that it might be sensible to change your lifestyle, if you do not meet current health authority guidelines. Our advice is to hit your endothelial cells as frequently as possible with brief intense bouts of exercise.

Anatomy of the endothelial cell layer (ECL)
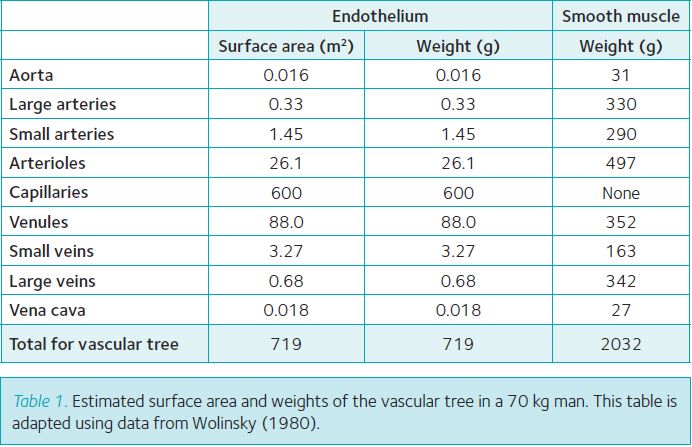
Every blood vessel in the human vasculature is covered on the luminal side with a continuous monolayer of endothelial cells (ECL). The total surface area of the ECL in an adult human has been estimated to cover >700 m2 and to have a weight of about 700 g (Table 1). This makes the ECL into one of the largest diffuse organs with a significant weight despite the 0.3 µm thickness. The ECL of arteries and veins together covers only about 6 m2 (Table 1), while the ECL of the microvasculature (arterioles, capillaries and venules) contributes 99% of the total surface area. Of the latter 85% (about 600 m2) is present in capillaries (Table 1). As skeletal muscle in a 70 kg lean physically active adult has a mass between 35 kg and 40 kg and has a higher capillary density than other tissues (with the exception of the heart), estimates are that at least 400 m2 of the ECL is present in the skeletal muscle microvasculature, again with the largest surface area in the dense network of capillaries (Wolinsky, 1980). The metabolic rationale behind this distribution is that the ECL in skeletal muscle capillaries is uniquely equipped for the transendothelial transport of nutrients, oxygen and hormones into the interstitial fluid that surrounds the muscle fibres, and that transport capacity depends on the available surface area (Fig. 1). It is assumed that only about 10% of the capillaries in skeletal muscle are perfused in the resting state with large increases in capillary recruitment occurring during high intensity aerobic exercise (Andersen & Saltin, 1977). This additional recruitment during exercise in combination with progressive increases in cardiac output and total muscle perfusion is required to ensure that the supply of blood borne fuels and oxygen meets the high energy demand of the contracting muscle.
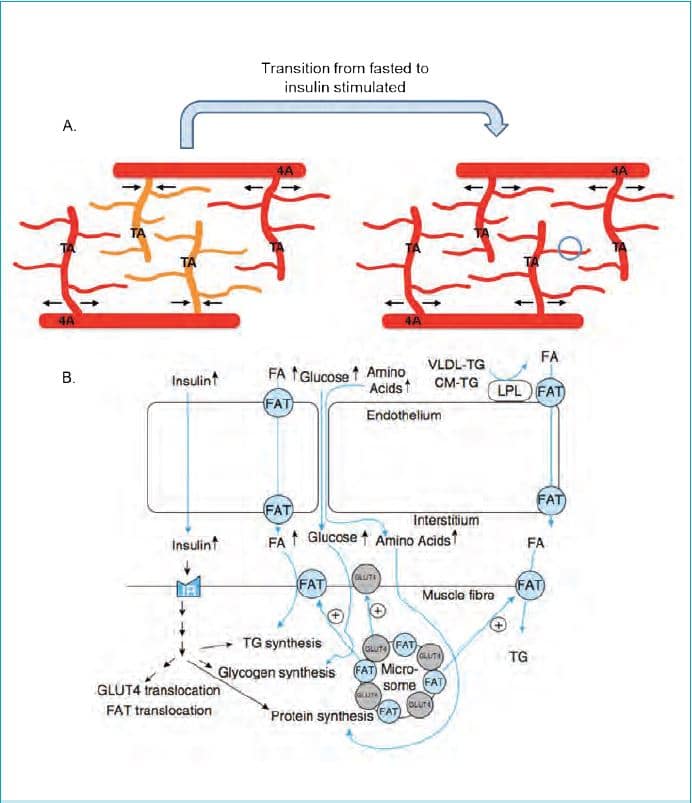
General functions of the endothelial cell layer (ECL)
A number of general functions of the ECL are common to all blood vessels (Bakker et al. 2009). The ECL by the nature of its location acts as a container for blood in the entire vascular tree. The ECL also acts as a selective barrier preventing the uncontrolled passage of pathogens, molecules and blood constituents that are not supposed to enter healthy cells in our organs and tissues. As such the ECL plays an important role in healthy individuals in preventing leucocytes from binding to the ECL and penetrating into the subendothelial layer where the leucocytes turn into foam cells and form atherosclerotic placques (Wagenmakers et al. 2006; Bakker et al. 2009). Other mechanisms act simultaneously to rapidly increase the permeability and available surface area of the ECL in periods of increased metabolic demand. This mostly applies to microvascular transport processes and several examples of this are given in the next section and in Fig. 1. The ECL has also been shown to prevent blood coagulation and the formation of a platelet thrombus and to produce regulators of fibrinolysis (Bakker et al. 2009). The luminal surface of the endothelium is covered with a brush like glycocalyx layer (glycoproteins and proteoglycans), which is 0.5 µm thick in capillaries and 4.5 µm in arteries (Reitsma et al. 2007; Weinbaum et al. 2007). In capillaries it is in direct contact with erythrocytes and leukocytes and acts as a lubricating layer for the narrow passage of these cells (with a diameter similar to the lumen of capillaries). The most important function of the glycocalyx is the translation of haemodynamic forces (shear forces exerted by flowing blood and individual blood cells passing through the narrow lumen of a terminal arteriole) into vasodilatory responses during exercise. As such mechanical forces exerted on the glycocalyx are likely to be early signalling events leading to eNOS activation and the molecular adaptation of the ECL to either increases or decreases in physical activity levels. The glycocalyx is also assumed to protect ECs against shear force-induced damage and in pathological states against damage caused by reactive oxygen species (Reitsma et al. 2007; Weinbaum et al. 2007).
Role of the ECL in insulin-mediated microvascular dilatation and muscle glucose uptake
Textbooks explaining the role of insulin in whole body glucose homeostasis traditionally assume that activation of the insulin signalling cascade in the skeletal muscle fibres is the main control mechanism (Fig. 2). However, there is now compelling evidence that insulin and glucose delivery to the skeletal muscle interstitium is a conditional early event in skeletal muscle glucose uptake (Coggins et al. 2001; Vincent et al. 2004; review of Barrett et al. 2009). Experiments using contrast-enhanced ultrasound (CEU), an established method which measures the volume of blood present in the muscle microvasculature (primarily in capillaries) and the microvascular blood flow, have shown that physiological increases in insulin lead to rapid increases in the microvascular blood volume in both rat and human skeletal muscle. In rats these increases occur as early as 5–10 min after the start of a physiological insulin infusion and precede both activation of the insulin signalling cascade in skeletal muscle and increases in glucose uptake by skeletal muscle, which are seen after 20–30 min (Vincent et al. 2004). This increase in microvascular blood volume has also been observed after ingestion of a mixed meal and during light exercise (Vincent et al. 2006). The increase in microvascular blood volume observed in these studies is taken to primarily reflect the recruitment of capillaries that were not perfused before. This conclusion is supported by Gudsbjornsdottir et al. (2003) using an independent method. The latter study made estimates of the permeability surface area product (PSA) for insulin and glucose in capillaries during an oral glucose tolerance test (OGTT) and physiological insulin infusion. The PSA went up twofold during the OGTT and 11-fold during the insulin infusion, with equal-fold increases seen for skeletal muscle glucose uptake.
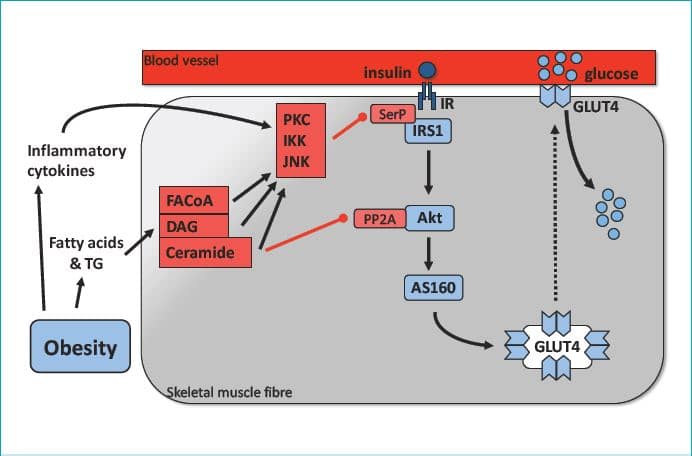
The molecular mechanisms leading to insulin-mediated recruitment of previously underperfused capillaries
The mechanisms responsible for insulin-mediated capillary recruitment have remained a puzzle for a long time. Important information has come from studies in cultured ECs (for references see Wagenmakers et al. 2006 and Muniyappa et al. 2007), which identified an endothelial insulin signalling cascade, activation of which leads to increased production of nitric oxide (NO, Fig. 3). NO produced by the ECL is a potent vasodilator acting upon smooth muscle cells (SMCs)) both in the macro- and in the microvasculature (Wagenmakers et al. 2006; Barrett et al. 2009). Insulin was found to activate the enzyme endothelial nitric oxide synthase (eNOS) by means of ser1176 phosphorylation in cultured rat ECs and ser1177 phosphorylation in cultured human ECs. eNOS converts the amino acid L-arginine to the products L-citrulline and NO. Other signals found to activate eNOS via ser1176/1177 phosphorylation are fluid shear forces exerted on a cultured EC monolayer and exposure of cultured ECs to vascular endothelial growth factor (VEGF). An important in vivo observation made by Vincent et al. (2004) was that pre-treatment of rats with the eNOS inhibitor L-NAME prevented the insulin induced increase in microvascular blood volume in skeletal muscle and reduced both glucose uptake and activation of the insulin signalling cascade in skeletal muscle. Collectively these observations led Rattigan et al. (2006) to propose that physiological increases in plasma insulin lead to serine phosphorylation of eNOS in the ECL of terminal arterioles in skeletal muscle with the increased NO production leading to relaxation of the VSM cells and dilatation of the terminal arterioles (Fig. 3). As one terminal arteriole supplies a series of capillaries with blood, this will then lead to recruitment of previously underperfused capillaries as explained in the top part of Fig. 1. Although to date, insulin-induced eNOS ser1177 phosphorylation has not been observed in the microvasculature of human skeletal muscle, it has been reported in the endothelium of large arteries.
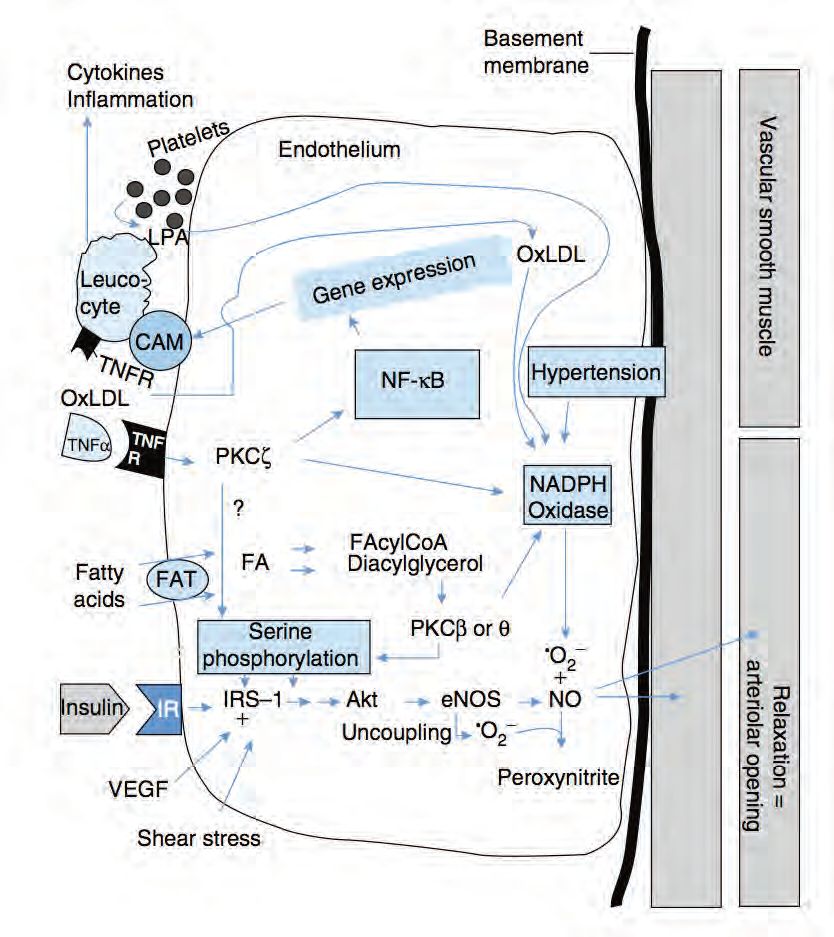
Impairments in the above mechanism lead to insulin resistance and reductions in angiogenesis in obesity and chronic disease
Impairments in insulin-mediated recruitment of skeletal muscle capillaries have been observed in obesity, metabolic syndrome and type 2 diabetes (Keske et al. 2009; Barrett et al. 2009). Experimental observations made in cultured endothelial cells and the ECL of larger arteries studied either in vivo or in arterial rings (for references see Wagenmakers et al. 2006) suggest that the underlying molecular mechanisms may involve a reduction in NO bioavailability leading to impaired vasodilatation and are summarised in Fig. 3 and its legend. Apart from these mechanisms the high levels of insulin that prevail in insulin resistant states also activate the MAPK pathway and lead to increases in endothelial expression of endothelin-1 (Muniyappa et al. 2007). Endothelin-1 is a potent vasoconstrictor, which will further increase the imbalance between vasodilatation and vasoconstriction, leading to a net reduction in insulin-mediated recruitment of skeletal muscle capillaries. Collectively these mechanisms may explain the substantial reductions that are seen in skeletal muscle glucose uptake during a hyperinsulinaemic euglycaemic clamp both in obese Zucker rats (Wallis et al. 2002) and in patients with type 2 diabetes (Gudsbjorndottir et al. 2004).
Wagenmakers et al. (2006) suggested that the metabolic impairments that lead to reductions in NO bioavailability (Fig. 3) in obesity, chronic disease and ageing may potentially also explain the reduction in the density of the capillary and microvascular network that is seen in these conditions. Evidence in support of this proposal is the experimental observation that eNOS inhibition with L-NNA prevented the angiogenic response to chronic electrical stimulation in rats in vivo (Hudlicka et al. 2006).
Novel assay to measure muscle microvascular endothelial enzymes
The mechanisms proposed in Fig. 3 are an extrapolation of previously published observations made in cultured endothelial cells and the ECL of large arteries exposed to insulin, VEGF and fluid shear flow either in vitro (arterial rings) or in vivo. The protein content and phosphorylation (activation) state of endothelial enzymes in these studies was measured in tissue extracts with Western blot analysis. As it is impossible to isolate capillaries and arterioles from percutaneous human skeletal muscle biopsies, Cocks et al. (2012) developed an immunofluoresence microscopy method in which cryosections of human vastus lateralis muscle were stained using antibodies targeting eNOS, eNOS phosphorylated at ser1177 and NOX2 (subunit of NAD(P)H oxidase). Quantification was achieved by analysing fluorescence intensity within the area that stained positive for the microvascular endothelium. This method in the meantime has shown that 1 h of cycling exercise at 65% VO2max significantly increased eNOS ser1177 phosphorylation (1.29 ± 0.05-fold change; n = 8 lean sedentary males). A pilot study in lean (LZR) and obese Zucker rats (OZR) also has shown that insulin stimulation during a hyperinsulinaemic euglycaemic clamp led to a significant increase in eNOS phosphorylation at ser1176 in the terminal arterioles of the anterior tibialis muscle of the LZR, while a significant decrease was seen in the OZR (Cocks & Wagenmakers, unpublished data).
Impact of various modes of exercise training on metabolic health of the ECL
Traditional endurance training (ET) is recognised as an efficient means to increase eNOS gene expression, protein content and NO production in feeding and resistance arteries (McAllister & Laughlin, 2006), thereby increasing the vasodilatory response to insulin (Barrett et al. 2009) and reducing the risk for the development of hypertension and atherosclerosis (McAllister & Laughlin, 2006). ET is also the traditional means to increase the production in skeletal muscle of VEGF, which stimulates angiogenesis via an NO-dependent signal. Recently sprint interval training (SIT; a special form of high intensity interval training (HIT) which requires an exercise cycle ergometer normally only present in specialised exercise laboratories) has received much attention as it was shown to elicit similar muscle metabolic (increases in activity of mitochondrial enzymes, aerobic exercise capacity, intramuscular triglyceride breakdown during exercise and insulin sensitivity) and macrovascular adaptations as ET, despite a marked reduction in time commitment (for references see Cocks et al. 2013 and Shepherd et al. 2013). Given that the most commonly cited barrier to physical activity is lack of time, it is thought that SIT and HIT may represent an effective strategy to stimulate exercise participation in sedentary individuals. SIT and other forms of HIT, which require access to regular gym equipment such as spinning bikes, may therefore provide a time efficient alternative (requiring only 3 sessions of 20–30 min per week versus 5 sessions of 40–60 min per week in traditional ET protocols) to increase muscle capillary density and protein content of eNOS. To test this hypothesis Cocks et al. (2013) have used the novel immunofluoresence microscopy method described above to compare the effects of 6 weeks of ET versus SIT. SIT was found to be as effective as ET in increasing muscle capillary density and insulin sensitivity, while SIT increased the skeletal muscle microvascular eNOS content significantly more (36%; P <0.05) than ET (14%).
Health advice to maintain a life-long healthy ECL
This article comes to the conclusion that the endothelial cell layer (ECL) of the skeletal muscle microvasculature plays a major role in the mechanisms that control skeletal muscle glucose uptake in health and disease (to include obesity, metabolic syndrome, type 2 diabetes and cardiovascular disease). Our recent study (Cocks et al. 2013) in lean, healthy, but sedentary young men, comes to the conclusion that the ECL of the skeletal muscle microvasculature is extremely sensitive to 6 weeks of ET and SIT with measurable increases in capillary density, eNOS protein content and insulin sensitivity. The current exercise guidelines of the WHO, American College of Sports Medicine and UK Department of Health are to participate for minimally 150 min per week in moderate intensity endurance exercise, which by many is regarded as being an unrealistic target. Therefore, our advice to the readers of this article who fail to meet this target despite trying hard is to hit the endothelial cell layer of your skeletal muscle microvessels with HIT to prevent impaired glucose tolerance and chronic disease in the future. The excuse that your job and personal life are so demanding that there is not enough time for exercise is no longer valid and the price paid in later life for not making time today will be very high.
References
Andersen P & Saltin B (1985). Maximal perfusion of skeletal muscle in man. J Physiol 366, 233–249.
Bakker W, Eringa EC, Sipkema P & van Hinsbergh VWM (2009). Endothelial dysfunction and diabetes: roles of hyperglycemia, impaired insulin signalling and obesity. Cell Tissue Res 335, 165–189.
Barrett EJ, Egglestone EM, Inyard AC, Wang H, Li G, Chai W & Liu Z (2009). The vascular actions of insulin control its delivery to muscle and regulate the rate-limiting step in skeletal muscle insulin action. Diabetologia 52, 752–764.
Cocks M, Shepherd SO, Shaw CS, Achten J, Costa ML, & Wagenmakers AJM (2012). Immunofluorescence microscopy to assess enzymes controlling nitric oxide availability and microvascular blood flow in muscle. Microcirculation 19, 642–651.
Cocks M, Shaw CS, Shepherd SO, Fisher JP, Ranasinghe AM, Barker TA, Tipton KD & Wagenmakers AJM (2013). Sprint interval training and endurance training are equally effective in increasing muscle microvascular density and eNOS content in sedentary males. J Physiol 591, 641–656.
Coggins M, Lindner J, Rattigan S, Jahn L, Fasy E, Kaul S & Barrett E (2001). Physiologic hyperinsulinemia enhances human skeletal muscle perfusion by capillary recruitment. Diabetes 50, 2682–2690.
Gudbjörnsdóttir S, Sjöstrand M, Strindberg L, Wahren J, Lönnroth P (2003). Direct measurements of the permeability surface area for insulin and glucose in human skeletal muscle. J Clin Endocrinol Metab 88, 4559–4564.
Gudsbjörndóttir S, Sjöstrand M, Strindberg L. & Lönnroth P (2005). Decreased muscle capillary permeability surface area in type 2 diabetic subjects. J Clin Endocrinol Metab 90, 1078–1082.
Hudlicka O, Brown MD, May S, Zakrzewicz A & Pries AR (2006). Changes in capillary shear stress in skeletal muscles exposed to long-term activity: role of nitric oxide. Microcirculation 13, 249–259.
Keske MA, Clerk LH, Price WJ, Jahn LA & Barrett EJ (2009). Obesity blunts microvascular recruitment in human forearm muscle after a mixed meal. Diabetes Care 32, 1672–1677.
McAllister RM & Laughlin MH (2006). Vascular nitric oxide: effects of physical activity, importance for health. Essays Biochem 42, 119-131.
Muniyappa R, Montagnani M, Koh KK & Quon MJ (2007). Cardiovascular actions of insulin. Endocr Rev 28, 463–491.
Naruse K, Rask-Madsen C, Takahara N, Ha SW, Suzuma K, Way KJ, Jacobs JRC, Clermont AC, Ueki K, Ohshiro Y, Zhang J, Goldfine AB & King GL (2006). Activation of vascular protein kinase C-b inhibits Akt-dependent endothelial nitric oxide synthase function in obesity-associated insulin resistance. Diabetes 55, 691–698.
Rattigan S, Bradley EA, Richards M & Clark MG (2006). Muscle metabolism and control of capillary blood flow: insulin and exercise. Essays Biochem 42, 133-144.
Reitsma S, Slaaf DW, Vink H, van Zandvoort MA & Oude Egbrink MG (2007). The endothelial glycocalyx: composition, functions and visualisation. Pflugers Arch 454, 345–359.
Shepherd SO, Cocks M, Tipton KD, Ranasinghe AM, Barker TA, Burniston JG, Wagenmakers AJM & Shaw CS (2013). Sprint interval and traditional endurance training increase net intramuscular triglyceride breakdown and expression of perilipin 2 and 5. J Physiol 591, 657–675.
Vincent MA, Clerk LH, Lindner JR, Klibanov AL, Clark MG, Rattigan S & Barrett EJ (2004). Microvascular recruitment is an early insulin effect that regulates skeletal muscle glucose uptake in vivo. Diabetes 53, 1418–1423.
Vincent MA, Clerk LH, Lindner JR, Price WJ, Jahn LA, Leong-Poi H & Barrett EJ (2006). Mixed meal and light exercise each recruit muscle capillaries in healthy humans. Am J Physiol Endocrinol Metab 290, E1191–E1197.
Wallis MG, Wheatley CM, Rattigan S, Barrett EJ, Clark ADH & Clark MG (2002). Insulin-mediated hemodynamic changes are impaired in muscle of Zucker obese rats. Diabetes 51, 3492–3498
Wagenmakers AJM, Van Riel NAW, Frenneaux MP & Stewart PM (2006). Integration of the metabolic and cardiovascular effects of exercise. Essays Biochem 42, 193–210.
Weinbaum S, Tarbell JM & Damiano ER (2007). The structure and function of the endothelial glycocalyx layer. Ann Rev Biomed Eng 9, 121–167.
Wolinsky H (1980). A proposal linking clearance of circulating lipoproteins to tissue metabolic activity as a basis for understanding atherogenesis. Circ Res 47, 301–311.
