
Physiology News Magazine
How does your gut grow?
The presence of food in the gut is a powerful driver of cell (and crypt) reproduction
Features
How does your gut grow?
The presence of food in the gut is a powerful driver of cell (and crypt) reproduction
Features
Robert A Goodlad
Histopathology Unit, Cancer Research UK, London
https://doi.org/10.36866/pn.53.27

In whatever tissue system you are interested, the gut is likely to either be the largest or the second largest. It is the main endocrine organ and immune organ and is also our second brain. The hindgut is a serious bacterial culture system and it is a strange thought that there are more bacterial cells in the colon than human cells in the entire body.
My main view of the gut is as a cell renewal system and one can argue that it is the only tissue in which to study proliferation and growth control mechanisms. This is due to its accessibility and its well organised anatomy with the proliferative and functional zones being neatly separated. The lining of the intestine divides rapidly, giving it great powers of renewal and of adaptation.
This adaptability means we can easily perturb the system and soon see it respond. One can thus profoundly increase intestinal mucosal cell division by overfeeding or cause proliferative rates to plummet by starvation. Starvation obviously reduces the content of the gut lumen and similar falls in proliferation can be obtained by feeding animals intravenously, as the animal is fed but the gut is starved. A less elaborate model is to feed animals on low residue (elemental) diets. With these diets the work of digestion is all done in the front of the gut so that the distal small intestine and colon atrophy.
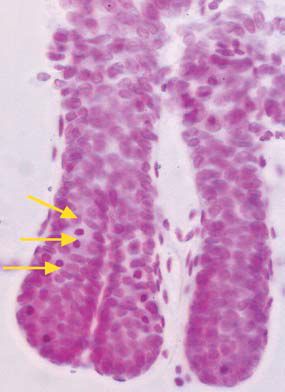
We have used the low residue diet in our recent paper in Experimental Physiology (Sasaki et al. 2003) to study the actions and interactions of two powerful growth factors, namely epidermal growth factor and keratinocyte growth factor. We used the crypt microdissection method (see Fig 1). Microdissection sounds very complicated, but is actually quite straightforward and is far quicker than scoring histological sections. One ‘downside’ of its productivity is that we are often tempted to quantify several sites of the gut! The method also avoids many of the problems associated with scoring sections and with simple proliferative or labelling indices, which we have shown on several occasions to miss significant events if both halves of these fractions change at the same time. For example we have shown that in intravenously fed rats the gut weight is dramatically reduced, as is crypt cell production; however, labelling indices showed no difference. This was a result of both the number of cells per crypt and the number of labelled cells decreasing together (Goodlad et al. 1992). If the labelling data was expressed as labelling per crypt the large proliferative changes become apparent, but it is far simpler to score mitotic figures or arrested metaphases directly in the ‘microdisected’ crypts. A further advantage of the method is that it can be used to quantify crypt fission (Fig. 2). Crypt fission is another way of altering intestinal mass, and although it is mainly seen in the young animal it persists throughout life and can play a role in adaptation following injury or trauma. It is also much increased in rodent and in human cancers (Wong et al. 2002).
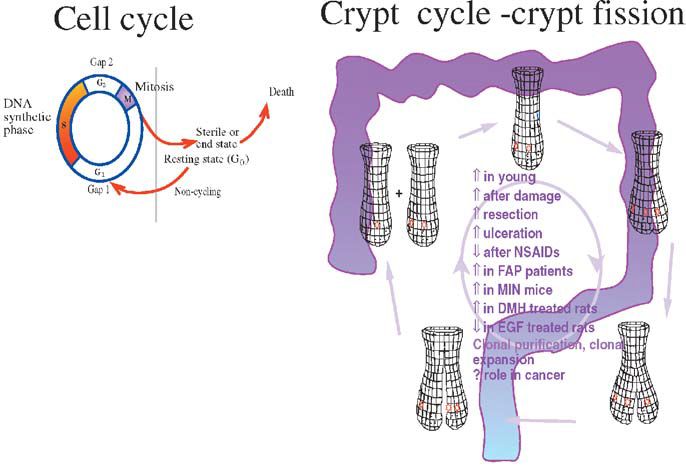
When mice are fed an elemental diet there is a large fall in proliferation and in crypt fission, especially in the distal small intestine and colon (Sasaki et al. 2002). This shows the importance of luminal contents for stimulating the gut. Such effects of ‘luminal nutrition’ may occur directly or more likely they are a consequence of ‘intestinal workload’ and several gut hormones and growth factors are likely to be involved. Diet induced atrophy can be very useful from an experimental point of view, as the effects of various trophic agents are more readily seen. In the Experimental Physiology paper we have used the model to see what happens when EGF and KGF are given alone or in combination. Both growth factors increased proliferation and reduced fission, but when given together the effects (on some sites) were interactive. The ability to alter cell renewal in the gut may be very important, as prevention of atrophy could be useful in several clinical conditions. Lowered proliferation in patients fed elemental diets can reduce mucosal integrity and some diets are already supplemented with ‘naturally occurring’ growth factors. Patients with short bowel syndrome, ulcers or inflammatory bowel disease, babies with damaged guts and those at risk of mucusitis due to chemotherapy or radiotherapy may all benefit from such therapy; however, cell proliferation is also a two edged sword, as excessive proliferation or fission may increase the growth of cancerous cells.
Of course, such experiments may be occurring every time we eat. We have used similar elemental diets or low fibre semisynthetic diets to look at the effects of starving or feeding the colon by altering the content of dietary fibre and similar colonic substrates. Low fibre diets reduce cell proliferation in the colon and dietary fibre or similar fermentable substrates dramatically increase cell proliferation, but only when the bacteria are present. Thus it is the breakdown of fibre by the bacteria to produce short chain fatty acids that stimulates cell division in the colon. Paradoxically these acids, especially butyric acid, seem to have the opposite effects on cells in vitro. Thus whether all ‘fibres’ are necessarily a good thing is still a matter of intense debate especially in the light of recent epidemiological findings. Diets that are naturally high in fibre are beneficial for many reasons, but recent attempts to broaden the definition of fibre and increase fibre intake through the use of these fermentable substrates could well be harmful (Goodlad 2001; Goodlad & Englyst, 2001).
References
Goodlad RA (2001). Dietary fibre and the risk of colorectal cancer. Gut 48, 587-589.
Goodlad RA & Englyst HN (2001). Redefining dietary fibre: potentially a recipe for disaster. Lancet 358, 1833-1834.
Goodlad RA, Lee CY & Wright NA (1992). Cell proliferation in the small intestine and colon of intravenously fed rats: effects of urogastrone-epidermal growth factor. Cell Prolif 25, 393-404.
Sasaki M, FitzGerald AJ, Grant G, Wright NA & Goodlad RA (2002). Lectins can reverse the distal intestinal atrophy associated with elemental diets in mice. Alimentary Pharmacology and Therapeutics 16, 633-643.
Sasaki M, FitzGerald AJ, Mandir N, Berlanga-Acosta J & Goodlad RA (2003). Keratinocyte growth factor and epidermal growth factor can reverse the intestinal atrophy associated with elemental diets in mice. Experimental Physiology 88, 261 –267.
Wong WM, Mandir N, Goodlad RA, Wong BC, Garcia SB, Lam SK & Wright NA (2002). Histogenesis of human colorectal adenomas and hyperplastic polyps: the role of cell proliferation and crypt fission. Gut 50, 212-217.
