
Physiology News Magazine
How ion channels and transporters affect metastasis
Cell migration is an important mechanism in metastasis. Ion transporters such as the Na+/H+ exchanger NHE1 control migration of tumour cells by, among other things, modulating cell adhesion. NHE1 creates a pH nanoenvironment at the cell surface that allows a coordinated formation and release of cell-matrix contacts during cell migration
Features
How ion channels and transporters affect metastasis
Cell migration is an important mechanism in metastasis. Ion transporters such as the Na+/H+ exchanger NHE1 control migration of tumour cells by, among other things, modulating cell adhesion. NHE1 creates a pH nanoenvironment at the cell surface that allows a coordinated formation and release of cell-matrix contacts during cell migration
Features
Christian Stock & Albrecht Schwab
Institute of Physiology II, The University of Münster, Münster, Germany
https://doi.org/10.36866/pn.62.19

Cell migration away from the primary tumour is a major step in metastasis. This multistep process requires sequential interaction between the invasive cell and the surrounding extracellular matrix as follows:
- migrating cells release matrix metalloproteinases in order to cleave their way through the dense, fibrous network of the extracellular matrix
- a directed reorganisation of the cytosekeleton, especially actin filaments, is needed for the continuous renewal of protrusive structures at the front of a motile cell
- the simultaneous action of the Na+/H+ exchanger NHE1, the Cl-/HCO3- exchanger AE2, the aquaporin AQP1 and possibly the Na+-HCO3 cotransporter NBC1 mediate local salt and water uptake at the leading edge of the lamellipodium which induces gradual cell swelling and causes the leading edge to grow (Schwab, 2001, Fig. 1)
- a coordinated formation and release of focal adhesion contacts to the extracellular matrix mediated by receptor molecules such as integrins enables the migrating cell to grab and loose its hold on the extracellular matrix.

Our interest is focused on the roles ion transporters and channels play in cell migration. One of these transporters whose role in cell migration is well studied is the Na+/H+ exchanger isoform NHE1. The primary task of the NHE1 is the regulation of intracellular pH and cell volume. Its housekeeping activity is of particular importance for many tumour cells. Insufficient tumour vascularization leads to a diminished O2 supply, so that anaerobic glycolysis leads to an accumulation of protons in the cytosol. Excess protons are extruded into the extracellular space by the NHE1 which then causes an acidification of the tumour tissue.
NHE1 activation enhances the invasiveness of tumour cells (Reshkin et al. 2000; Cardone et al. 2005) while blocking NHE1 inhibits cell migration in several cell types. The fact that NHE1 and integrin dimers colocalize at the focal adhesion contacts led us to hypothesize that NHE1 creates a pH nanoenvironment at the surface of the plasma membrane which then affects cell migration.
To prove this hypothesis we studied the migration of human melanoma cells that utilize a2b1 integrins for establishing reversible cell-matrix contacts (Maaser et al. 1999). Migration of these cells depends on both NHE1 activity and the extracellular proton concentration (Stock et al. 2005). Cells reach their maximum motility at extracellular pH values of about 7.0 to 7.2. They hardly migrate at pH 6.6 or 7.5, when NHE1 is inhibited, or when NHE1 activitiy is stimulated by loading cells with protons. Under these different conditions characteristic changes in intracellular pH are observed. The changes in the intracellular pH, however, cannot account for the changes in migratory behaviour. Migration instead correlates with the strength of cell-matrix interactions, as adhesion is strongest at an extracellular pH of 6.6 and weakens at basic extracellular pH values, upon NHE inhibition or upon blockage of the integrin α2β1.
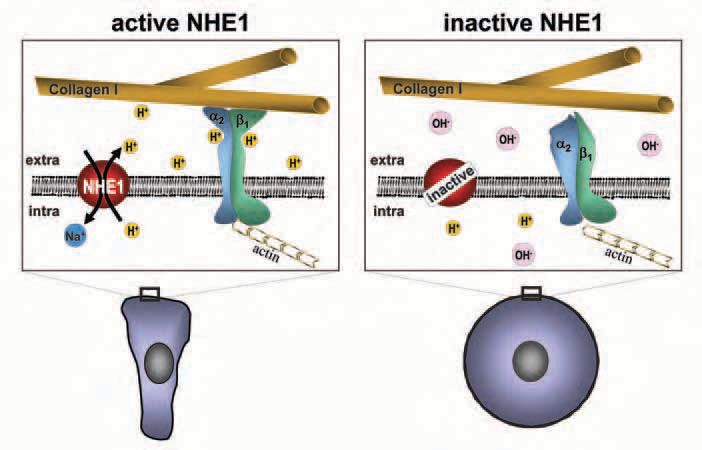
At this point a direct interrelation between NHE1 activity, extracellular proton concentration, cell migration and adhesion begins to appear (Fig. 2). The extracellular protons and NHE1 activity affect migration of human melanoma cells by modulating cellmatrix interactions. Migration is hindered when the interaction is too strong (acidic extracellular pH) or too weak (alkaline extracellular pH or NHE1 inhibition).
Implications
Ion transporters such as NHE1 might be potential targets for chemotherapeutics within the scope of the treatment of cancer.
For instance NHE1 inhibitors such as cariporide (also known as HOE642), have been shown to have low levels of toxicity, so that they might be useful as anti-cancer agents. Cariporide has been shown to have antiproliferative activity in vitro, and long-term treatment of rats with this drug prevents them from dying of cancer. Only time and more research will tell if NHE1 will really provide a new angle of attack on cancer, but the signs are promising.
Acknowledgements
This work was supported by grants from the Deutsche Forschungs gemeinschaft (Schw 407/7-1, 407/9-1 and Re 1284/2-1).
References
Cardone RA, Casavola V & Reshkin SJ (2005). The role of disturbed pH dynamics and the Na+/H+ exchanger in metastasis. Nature Rev Cancer, 5, 786-795.
Maaser K, Wolf K, Klein CE, Niggemann B, Zänker KS, Bröcker E-B & Friedl P (1999). Functional hierarchy of simultaneously expressed adhesion receptors: integrin a2b1 but not CD44 mediates MV3 melanoma cell migratiojn and matrix reorganization within threedimensional hyaluron-containing collagen matrices. Mol Biol Cell 10, 3067-3079.
Reshkin SJ, Bellizzi A, Albarini V, Guerra L, Tommasino M, Paradiso A & Casavola V (2000). Phosphoinositide 3-kinase is involved in the tumor-specific activation of human breast cancer cell Na+/H+ exchange, motility, and invasion induced by serum deprivation. J Biol Chem 275, 5361-5369.
Schwab A (2001). Function and spatial distribution of ion channels and transporters in cell migration. Am J Physiol Renal Physiol 280, F739-F747.
Stock C, Gassner B, Hauck CR et al. (2005). Migration of human melanoma cells depends on extracellular pH and Na+/H+ exchange. J Physiol 567, 225-238.
