
Physiology News Magazine
Hyaluronan, the guardian of joints
Most body fluid cavities are sealed by a tight epithelium or mesothelium, whereas the joint lining is almost devoid of cell junctions, 20-30% of its interface being ‘naked’ intercellular matrix. How then is the hyaluronan-rich, lubricating synovial fluid retained? When intra-articular pressure is raised (e.g. by flexion), ultrafiltration of the tangled hyaluronan chains by the synovial extracellular matrix creates a dynamic, reversible concentration polarisation layer with sufficient osmotic pressure to oppose further fluid loss. In addition HA reflection also raises the fluid’s viscosity and reduces the energy-expensive replacement synthesis rate
Features
Hyaluronan, the guardian of joints
Most body fluid cavities are sealed by a tight epithelium or mesothelium, whereas the joint lining is almost devoid of cell junctions, 20-30% of its interface being ‘naked’ intercellular matrix. How then is the hyaluronan-rich, lubricating synovial fluid retained? When intra-articular pressure is raised (e.g. by flexion), ultrafiltration of the tangled hyaluronan chains by the synovial extracellular matrix creates a dynamic, reversible concentration polarisation layer with sufficient osmotic pressure to oppose further fluid loss. In addition HA reflection also raises the fluid’s viscosity and reduces the energy-expensive replacement synthesis rate
Features
J R Levick
Physiology, Basic Medical Sciences, St. George’s Hospital Medical School, Cranmer Terrace, London SW17 0RE.
https://doi.org/10.36866/pn.67.18
Joint diseases lack the dramatic, life-threatening quality of cardiac disease, which may be why synovial joints have been largely neglected by physiologists. Or perhaps the neglect is due to the seeming simplicity of joints; after all, where is the intellectual challenge in a door hinge? This was very much my own response when joint physiology was mooted as a post-doctoral research area by my supervisor, Charles Michel. Thirty years on the naivety of this response is clear, for the apparent simplicity of joint function is achieved through a complex interplay of physiological, biophysical and biochemical mechanisms. This article describes one aspect of this, namely how joints manage to retain their lubricating fluid even though all the components of the fluid can, to a greater or lesser degree, seep out through the cavity lining.
Synovial fluid for lubrication and cartilage nutrition
Synovial joints contain a microscopically thin layer of highly viscous synovial fluid with two primary functions (Fig. 1). First, it transports glucose, amino acids and oxygen by convection-diffusion from the synovium to the articular cartilage, which is avascular but cellular and metabolically active. This mode of nutrition limits the thickness of viable articular cartilage to a few millimetres (Zhou et al. 2004). Second, synovial fluid lubricates the moving surfaces, limiting mechanical inflammation and reducing wear of not only the weight-bearing cartilage-on-cartilage interface but also cartilage-on-synovium and synovium-on-synovium interfaces. The lubricants include the high-load, boundary lubricant lubricin (glycoprotein) and the low-load, hydrodynamic lubricant hyaluronan (see below).

Synovium produces synovial fluid
Synovial fluid is produced by the vascular joint lining or synovium, as William Hunter (surgeon-obstetrician, St George’s Hospital) recognized in 1743. Synovium is a thin, discontinuous layer of mesoderm-derived cells bounding the joint cavity. Unlike the epithelium or mesothelium lining more familiar cavities such as the cerebrospinal space and pleural cavity, synovial cells form few junctions and are separated by µm-wide, matrix-filled gaps. As a result 20-30% of the fluid interface is ‘naked’ extracellular matrix (ECM) (Fig. 1). To cope with the inherent leakiness of this structure, a novel way of regulating the joint fluid content has evolved, involving the creation of a transient, reversible obstacle to fluid outflow (but not inflow) – a point we will return to. The water, nutrients, electrolytes and plasma proteins of synovial fluid come from the capillaries a few µm below the surface in accordance with established principles of capillary physiology (Levick, 1995). The highly water-permeable fenestrations of these capillaries are, appropriately, grouped on the side facing the joint cavity. The synovial lining cells themselves comprise 2/3rd fibroblast-like synoviocytes (FLS) and 1/3rd macrophages. The wide intercellular spaces are the sole drainage pathway from the joint cavity, allowing a turnover of fluid that has entered the cavity from the capillary fenestrations.
The FLS secrete the lubricants hyaluronan (HA) and lubricin into the plasma ultrafiltrate to create the highly viscous synovial fluid (syn-ovium refers to the egg-white consistency).
Joint angle affects intra-articular pressure, hence fluid turnover
Synovial fluid pressure varies with joint angle, being low in extension (a few cmH2O subatmospheric) and higher in flexion (2-5cmH2O). As a result capillary ultrafiltrate enters the cavity during extension and drains out through the interstitial pathway into subsynovial lymphatics during flexion. Joint movement thus acts as a pump to promote synovial fluid turnover. But here we meet a puzzle – when the turnover time is measured in rabbit and non-arthritic human knees, the turnover time for synovial fluid water and albumin is ∼2 h, whereas that for synovial fluid hyaluronan (calculated as mass/secretion rate) is 12-36 h. This indicates that hyaluronan may be selectively retained inside the cavity –with profound consequences for fluid exchange, as we shall see.
The hyaluronan molecule –more voluminous than a virus
Hyaluronan is a ∼3-6 million Dalton unbranched polymer of repeating acetyl glucosamine-glucuronic acid disaccharides. It is ‘spun’ from the FLS plasmalemma by the transmembrane enzyme HAS2. The µm-long chain adopts an extended, random coil configuration encompassing a vast, hydrated domain of radius100-200 nm (cf. albumin 3.6 nm). The resulting high, shear-dependent viscosity is enhanced further by overlap of adjacent molecular domains at concentrations of >1mg ml-l. HA is a universal component of ECM, including synovial ECM (0.8mg per ml extrafibrillar space), but its concentration in synovial fluid is particularly high, 2-4 mg ml-1 in human and rabbit knees. Both the concentration and chain length are reduced in arthritic effusions, lowering the viscosity.
Fluid leaks out from flexed joints
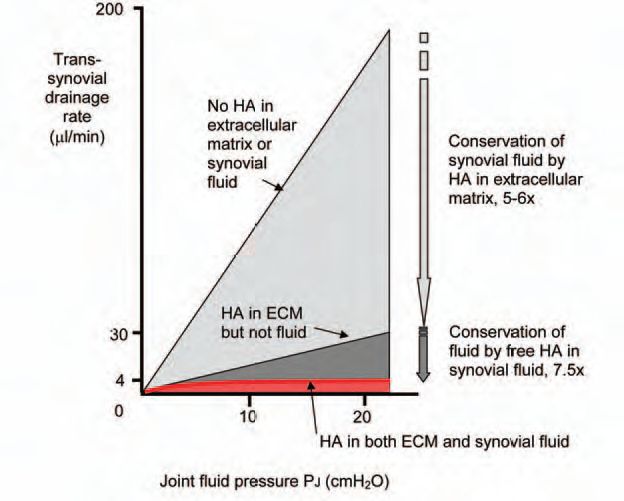
Trans-synovial fluid movement has been studied chiefly in the cannulated knees of anaesthetized rabbits. When the cavity is infused with physiological saline or albumin solution, trans-synovial fluid escape rate increases markedly with pressure (Fig. 2). The reader’s knee and hip joints have probably been in a flexed position for some time while reading this article, raising the intra-articular pressure and fluid loss. There is only ∼50-100 µl synovial fluid in a rabbit knee and 500-1000 µl in a non-arthritic human knee, so how do we avoid squeezing out this tiny volume during flexion? The answer seems to lie largely in the action of HA at two locations – the synovial lining ECM and the synovial fluid.
Extracellular matrix HA dramatically increases hydraulic resistance
When the soluble HA is washed out of the joint cavity experimentally, plots of trans-synovial flow-v-pressure show that the synovial drainage pathway, the ECM, has a substantial hydraulic resistance (Fig. 2, middle line). This has been confirmed using a servo-null micropipette to probe the trans-synovial pressure gradients. This high resistance underpins the fluid-encapsulating role of synovium. If the ECM is depleted of bound HA using Streptomyces hyaluronidase, the hydraulic permeability increase 5-10 fold, showing that matrix HA plays a key role in maintaining a low hydraulic permeability (Fig. 2, upper line). The magnitude of the effect is remarkable because HA is only a minor component of the matrix (0.3 mg (g tissue)-1). It may be that the µm-long HA molecule serves as the ‘string’ to which the other, major matrix components such as proteoglycans are attached (string of beads analogy). Cutting the string disrupts the matrix organization and hence its hydraulic resistance.
Synovial fluid HA buffers fluid outflow
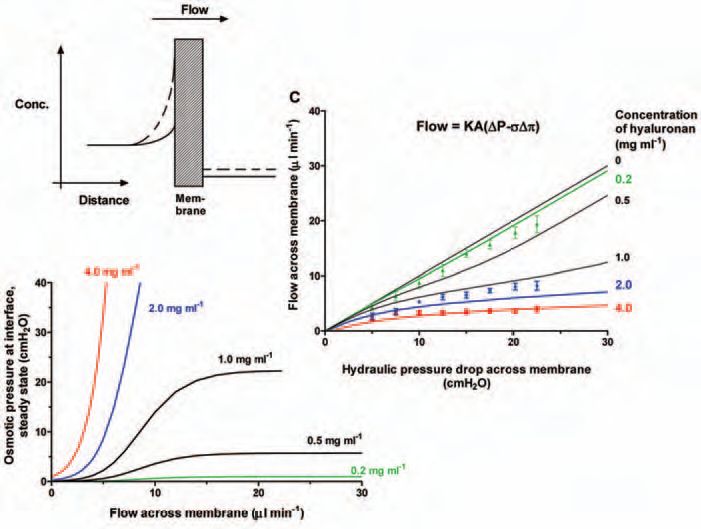
The free HA in synovial fluid causes a second major reduction in joint fluid escape, though not through its viscosity. When a HA solution is infused into the rabbit knee cavity, trans-synovial flow no longer increases steeply with pressure. Raising the intra-articular pressure now causes such small increases in fluid loss that the relation is almost a plateau (Fig. 2, bottom curve). This buffering of outflow depends on the HA concentration and molecular size and is reversed by HA washout. It also depends on the synovial ECM, being abolished by a collagen-sparing protease, chymopapain.
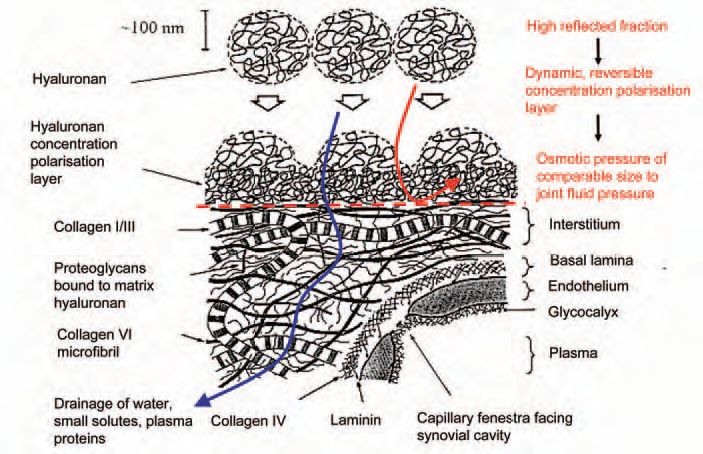
The quasi-plateau shows that the opposition to trans-synovial filtration increases in proportion to the applied pressure, and this led to the idea of outflow buffering through concentration polarisation. The idea is that the vast HA molecules experience partial ultrafiltration (molecular reflection) during initial filtration at the synovial ECM surface, creating a concentrated HA ‘filter-cake’ at the interface (Fig. 3). The osmotic pressure of this layer provides the force that opposes filtration. The greater the filtration pressure, the more concentrated the layer and the greater the osmotic force countering fluid escape – the hydraulic equivalent of a rectifying ion channel. Fluid can still enter the joint from the capillaries without hindrance, but its egress over a much bigger surface area is hindered by HA concentration polarisation.
Extracellular matrix as a molecular sieve
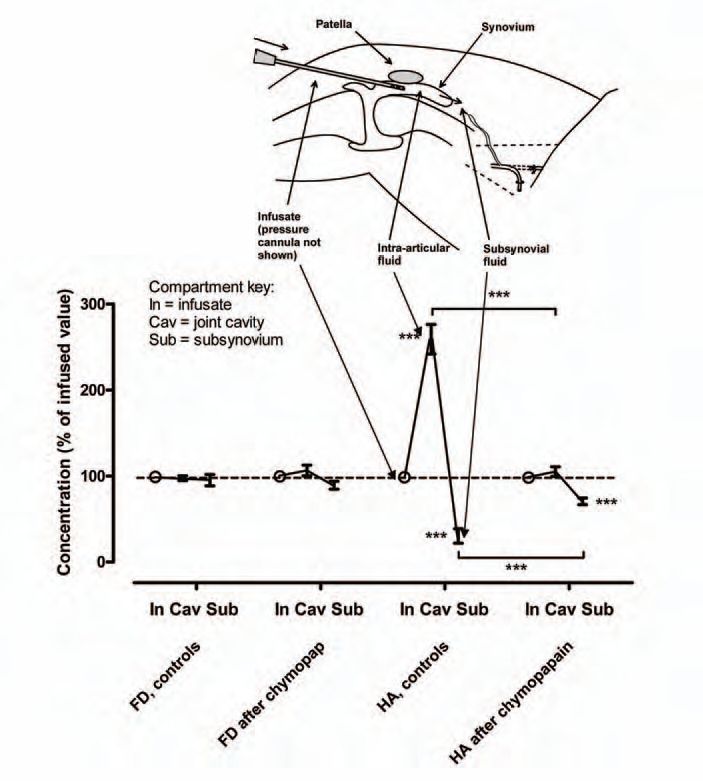
Does the joint lining reflect HA, as postulated above? When HA solutions were infused into anaesthetized rabbit knee joints and filtered under pressure across the joint lining, the intra-articular HA concentration increased and the subsynovial and lymph HA concentrations decreased, relative to the infusate (Fig. 4), indicating molecular ultrafiltration. Moreover, the proportion of HA molecules reflected fell with increasing filtration rates, a phenomenon characteristic of concentration polarisation (Lu et al. 2004). The reflected fraction depends on HA concentration and molecular size, just like the outflow buffering curves (Fig. 5). Depending on the conditions >90% of HA molecules are reflected and retained in the cavity. Since the cells lack tight junctions, the ECM must be the molecular sieve. In keeping with this, proteoglycan depletion by chymopapain almost completely abolishes HA reflection by rabbit synovium (Fig. 4) and increases the calculated interstitial pore radius from 33-59 nm to 192-336 nm.
The concentration polarisation and outflow buffering can be modelled mathematically as a quasi-steady state phenomenon (providing a simple, analytical solution) or more accurately as a non-steady state phenomenon (requiring computer simulation). Using experiment-derived parameters the models provide a good fit to the HA reflection results and outflow buffering curves over a range of HA concentrations (Fig. 5). The non-steady state model can be extended to describe the closed joint, where the boundary conditions differ from the open-ended infusion system used experimentally.
HA reflection and HA secretion
HA reflection has a wider importance besides its role as a guardian of joint fluid. Concentration polarisation boosts local viscous lubrication. Also, reflection prolongs the intra-articular working life of HA by an order of magnitude, and thus conserves energy-expensive replacement by synthesis. HA synthesis is a current growth point in the field. It is clear that mechano-sensitive regulatory pathways exist that couple HA secretion to joint usage; and, at least in culture, a Ca2+-PKCα-ERK1/2 pathway contributes to stretch-stimulated HA secretion (Momberger et al. 2006). The mechanosensitive, ERK-mediated HA production is also crucial to joint cavitation during embryogenesis (Lewthwaite et al. 2006).
Implications for arthritis
Arthritic joints contains a reduced concentration of HA of reduced chain length. As a result, the fluid viscosity is reduced and outflow buffering is lost (Fig. 5). Metalloproteinase activity is high, so HA loss is presumably increased, which may help explain the high plasma HA levels seen in rheuma-toid arthritis. Loss of outflow buffering will increase the fluid escape rate, helping to offset the increased fluid input from inflamed synovial microvessels (joint effusion). This ‘beneficial’ effect, however, is at the expense of increased loss of lubricant and the formation of periarticular oedema, which probably causes the characteristic morning stiffness of arthritis. The subsynovial oedema is due to poor coupling between subsynovial lymph drainage and trans-synovial filtration rate.
Intra-articular injections of HA are now a popular form of treatment for osteoarthritis in the USA and Japan. There is evidence of moderate benefit in moderately severe disease, but the mechanism of action over weeks/months is by no means clear.
Summary
The joint cavity lacks a tight cellular lining. Instead a biophysical hydraulic ‘rectifier’ has evolved, namely a reversible HA concentration polarisation layer. This greatly slows the fluid loss from the cavity through the extensive, relatively fast-draining ECM pathway in flexed joints (higher intra-articular pressure), while allowing unimpeded, slow plasma ultrafiltration into the cavity from superficial fenestrated capillaries in extended joints (low intra-articular pressure). Thus HA concentration polarisation compensates for the absence of the ion pump-based regulatory mechanisms found in many other fluid cavities; and reduces by an order of magnitude the need for replacement HA synthesis.
References
Lu YL, Levick JR & Wang W (2004). Concentration polarisation of hyaluronan on the surface of the synovial lining of infused joints. J Physiol 61, 559-573
Lu YL, Levick JR & Wang W (2005). Synovial fluid retention in pressurised joint cavities is achieved by hyaluronan concentration. Microcirculation 12, 581-595.
Levick.JR (1995). Microvascular architecture and exchange in synovial joints. Microcirculation 2, 217-233.
Lewthwaite JC, Bastow ER, Lamb KJ, Blenis J, Wheeler-Jones CPD & Pitsillides AA (2006). A specific mechanomodulatory role for p38 MAPK in embryonic joint articular surface cell MEK-ERK pathway regulation. J Biol Chem 281, 11011-11018.
Momberger TS, Levick JR & Mason RM (2006). Mechanosensitive synoviocytes; a Ca2+ -PKCα-MAP kinase pathway contributes to stretch-induced hyaluronan synthesis in vitro. Matrix Biology 25, 306-316.
Sabaratnam S, Coleman PC, Mason RM & Levick JR. (2007). Interstitial matrix proteins determine hyaluronan reflection and fluid retention in rabbit joints; effect of protease. J Physiol 578, 291-299.
Zhou S, Cui Z & Urban JPG (2004). Factors influencing the oxygen concentration gradient from the synovial surface of articular cartilage to the cartilage-bone interface. A modelling study. Arthritis and Rheumatism 50, 3915-3924.
