Physiology News Magazine
Imaging tumour biochemistry in vivo
Developing next generation tools for cancer diagnosis, monitoring of therapeutic response and detection of drug resistance
Features
Imaging tumour biochemistry in vivo
Developing next generation tools for cancer diagnosis, monitoring of therapeutic response and detection of drug resistance
Features
Tim Witney
Centre for Advanced Biomedical Imaging (CABI), UCL, London, UK
https://doi.org/10.36866/pn.104.30
Advances in modern-day medicine and the associated increase in life-span has resulted in an increased prevalence of age-related diseases, such as cancer. Despite substantial funding and some exciting breakthroughs, we are still a long way from turning this debilitating disease into a chronic illness. In many cases, we lack the tools to detect the disease at an early stage, where patients are most likely to respond to therapy. Moreover, in the clinic there is still a long way to go before the realisation of precision medicine, where patients receive the drug that is tailored to their individual disease. Through the development of advanced imaging techniques we aim to make precision medicine a reality.
|
|
Centre for Advanced Biomedical Imaging (CABI), UCL, London, UK |
My group is interested in creating new imaging agents and techniques to understand cancer growth, development and cell death. Imaging provides the ability to study these processes in living organisms with unrivalled temporal and spatial resolution, without the need for invasive biopsy sampling. With imaging, we are not limited to studying a snap-shot of a small section from the primary tumour, but can interrogate the cancer in its entirety, including tumours that may have metastasised to distant regions in the body. Importantly, we can study these processes in people and not just in animal models of cancer. This is enormously powerful and is what gets me so excited about being in this field.
As a biochemist by training, I’ve always been particularly interested in how cancer cells alter the way they consume nutrients in order to grow at such a rapid pace and outcompete surrounding normal tissue. Through the development of novel imaging tools, we can probe cancer biochemistry in living organisms. In hospitals throughout the world, cancer is routinely detected and monitored using a radioactive version of glucose, known as fluorodeoxyglucose, or FDG for short. Using an imaging method known as positron emission tomography (PET), we can map in 3D where the radioactive glucose is in the body. Tumours take up a lot of glucose in order to maintain their highly proliferative state, and so ‘light up’ in the PET scan. Using other radiolabelled molecules, we have been able to look not only at glucose utilisation, but explore how tumour cells use lipids as an alternative energy source, store glucose for a rainy day as glycogen, and probe the activity of key enzymes that regulate these processes.
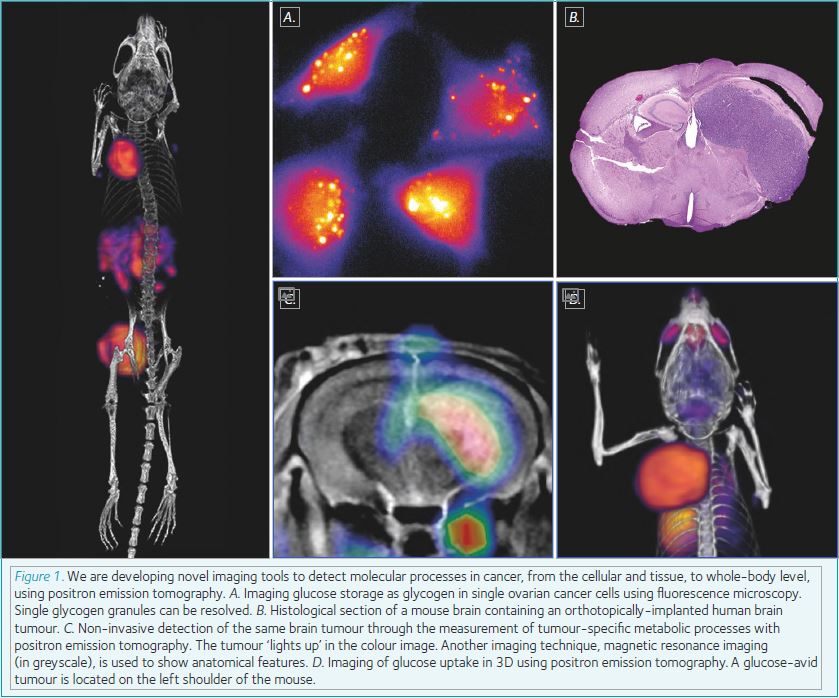
More recently, my research has shifted to focus on the pressing issue of drug resistance in cancer. Resistance to chemotherapy and molecularly-targeted therapies provides a major hurdle for cancer treatment, due to the underlying genetic and biochemical heterogeneity of tumours. Despite intensive research, the field has struggled to provide a solution for sensitive and specific molecular imaging of tumour response and resistance to therapy. We are looking at methods that cancer cells employ to resist traditional therapies, such as altered metabolism, and are actively pursuing these biomarkers as potential targets for imaging. A new diagnostic imaging test that can predict drug resistance will enable patient stratification, enabling the clinician to select an alternate therapy for the individual patient using a precision medicine approach. Where no suitable treatment options are available, patients with resistant disease will no longer receive inappropriate second-line treatment, thus avoiding associated side effects; resulting in substantially improved quality of life and the opportunity to initiate palliative care at an earlier stage.
Imaging probe development is an expensive process, requiring a multidisciplinary approach that spans the disciplines of the biomedical and physical sciences. I am lucky enough to work in a Centre that lives and breathes collaborative science, and have the support of the Wellcome Trust and the Royal Society. Imaging has shown to play a vital role in patient management, but we have only scratched the surface of what can be achieved. It is my hope that the next generation of imaging tools and techniques will lead to a better understanding of cancer and how we can keep it at bay.
Tim is a Group Leader in the Centre for Advanced Biomedical Imaging (CABI) at University College London (UCL), Wellcome Trust/Royal Society Sir Henry Dale Fellow and UCL Excellence Fellow.