Physiology News Magazine
Implications of demyelination for the structure and function of the neuromuscular junction
Recent studies of neuromuscular junctions in animal models of demyelinating disease suggest that glial-axonal signalling plays a crucial role in stablising synaptic form and function.
Features
Implications of demyelination for the structure and function of the neuromuscular junction
Recent studies of neuromuscular junctions in animal models of demyelinating disease suggest that glial-axonal signalling plays a crucial role in stablising synaptic form and function.
Features
Felipe Court
University of Edinburgh, Department of Neuroscience and Preclinical Veterinary Sciences
https://doi.org/10.36866/pn.47.12

Charcot-Marie-Tooth (CMT) diseases comprise a group of hereditary neuropathies heterogeneous in their clinical and molecular features. They were first described by Charcot and Marie, in France, and Tooth, in England, in 1886. The clinical phenotype of CMT, although showing considerable variability, is characterised by progressive muscle weakness and distal sensory dysfunction. Several genes have been associated with CMT and the original classification, mainly based on the disease phenotypic expression, has been reformulated; autosomal dominant demyelinating forms are referred as CMT1 and CMT3 and recessive demyelinated as CMT4. The CMT2 subclass represents those related to axonal gene mutations. In addition, a number of animal models representing different forms of CMT have been generated by genetic engineering or have arisen by spontaneous mutation. These are often excellent models of the correspondent human syndrome; hence they provide valuable information about disease mechanisms.
Aspects of the research in Peter Brophy’s lab focus on CMT conditions produced by defects in genes associated with Schwann cells, the myelin- forming cells of the peripheral nervous system. Specifically, the lab is interested in the effects of protein disruption on the formation and maintenance of myelin. Recently, axonal degeneration has been recognised as an indirect consequence of demyelinating conditions. This fact emphasises the dependency of the axon on its associated myelin forming cells and opens a valuable way to unveil the biology associated with axon-Schwann cell signalling. In addition, the recognition that axonal damage is not only an integral part of myelin disorders but also a major contribution to the pathology of the disease, may redirect the strategy of future therapeutic approaches.
My Communication to the Physiological Society at the Bristol meeting last year (*1) presented results of experiments I carried out in Richard Ribchester’s lab, relating to the involvement of neuromuscular junctions in neuropathic changes accompanying demyelination. Our initial aim was to seek possible changes in the functional and structural characteristics of the neuromuscular synapse as a consequence of demyelinating disorders. In so doing we hoped to evaluate the contribution of motor end-plate plasticity to the phenotype of demyelinating conditions. The rationale for this was the fact that axonal degeneration, described in several demyelinating forms of CMT conditions, could be a consequence not only of the lack of trophic support from neighbouring Schwann cells, but might also be elicited by the interruption of trophic signals originated in the muscle fibre, some of them known to be critical for neuronal survival. We used the periaxin knock-out mouse (Prx-/-), an animal model of CMT (autosomal recessive demyelinating, classified as CMT4F) to explore the consequences of a demyelinating condition in the neuromuscular junction.
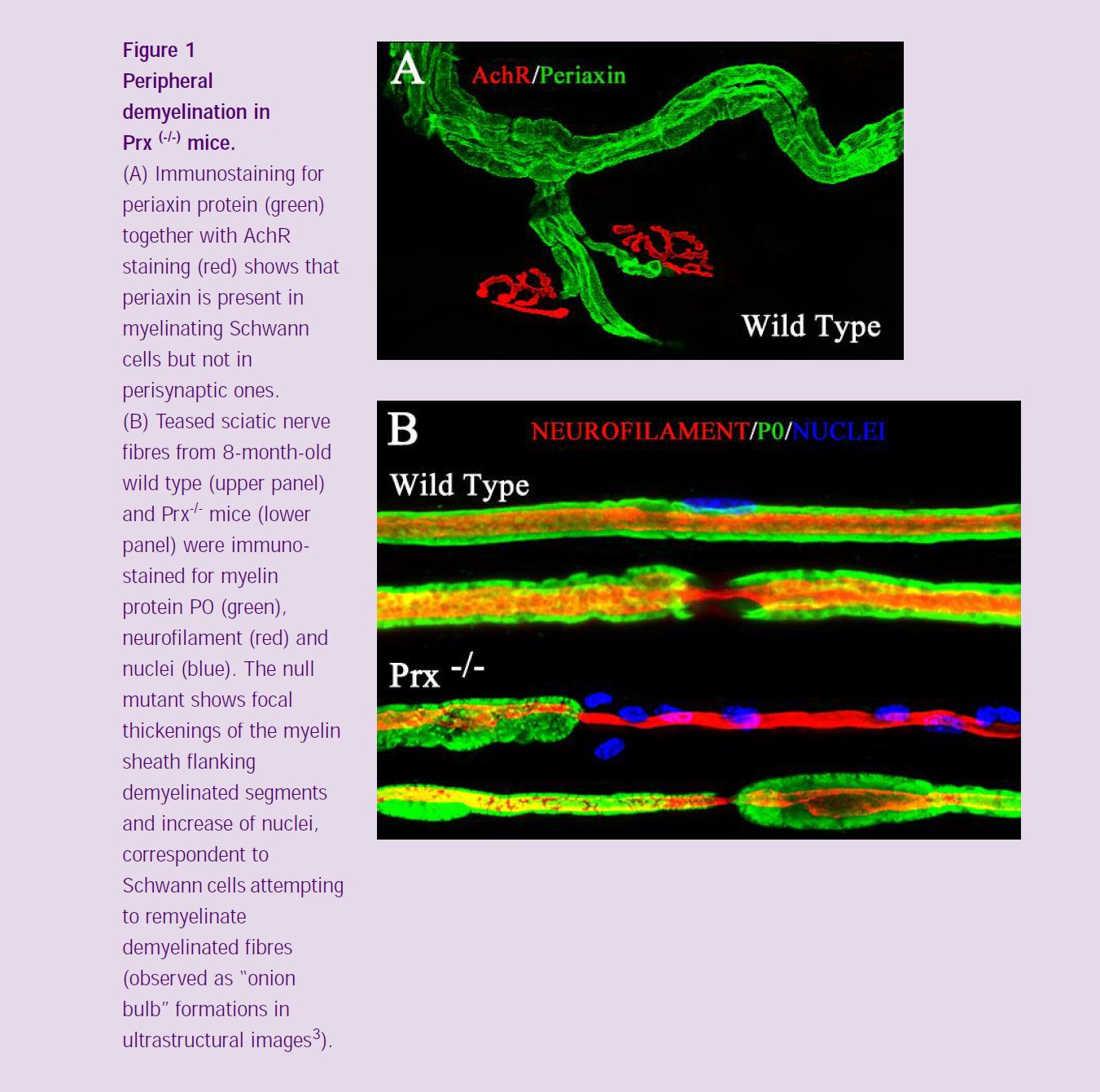
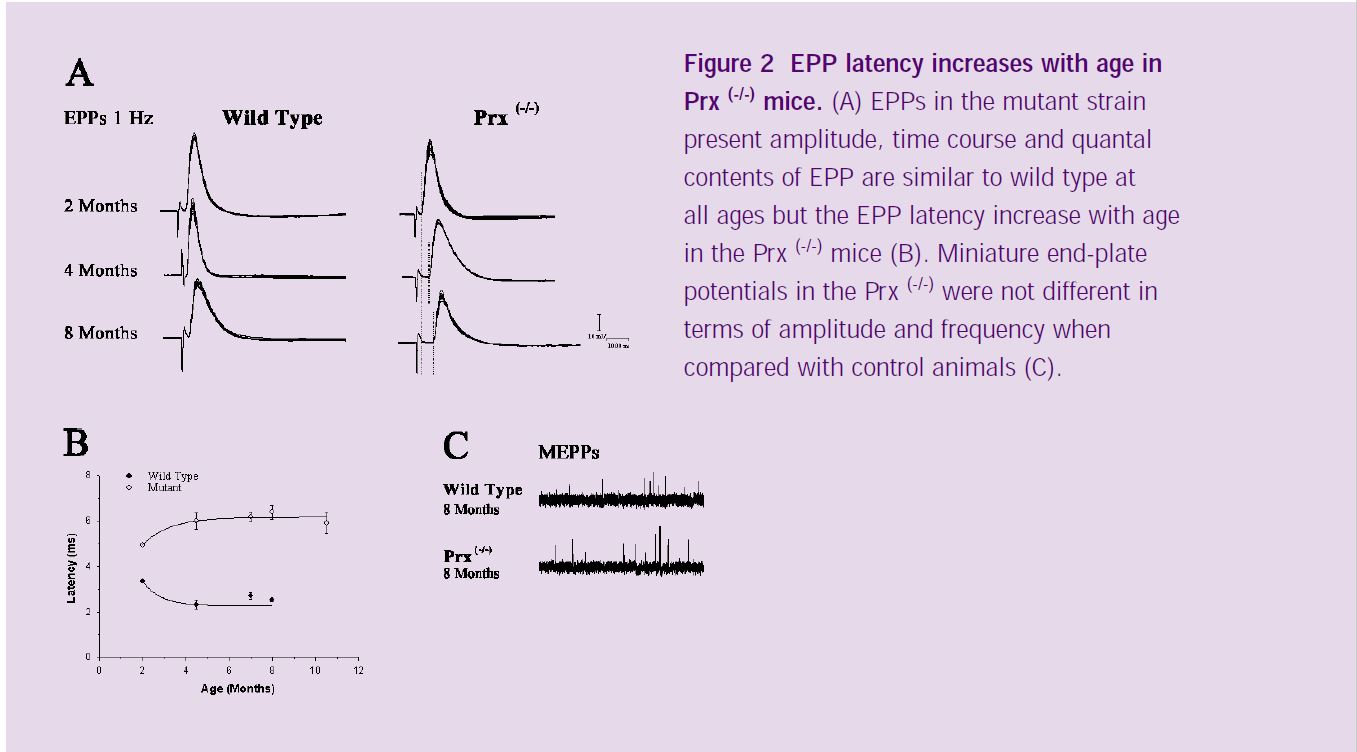
The expression of the Periaxin proteins (L- and S-periaxin) is restricted to myelinating Schwann cells in the peripheral nervous system. Prx-/- mice initially produce compact myelin, but later demyelinate due to disruption of a novel dystroglycan complex (*2) (Figure 1). Phenotypically, the mice show tremor, inappropriate clasping reflexes, reduced peripheral nerve conduction velocity and pain behaviour (*3). I used intracellular recording from muscle fibers to evaluate the physiological characteristics of neuromuscular transmission in the Prx-/- mice and immunohistochemistry together with confocal microscopy in order to compare the morphological features of axons, myelinating and terminal Schwann cells, and postsynaptic apparatus of Prx-/- and control mice. End-plate potential (EPPs) recordings at low frequency of stimulation (1 Hz) revealed that most of the mutant junctions showed normal synaptic responses, with amplitude, time course and quantal contents of EPP similar to wild type at all ages. In addition, miniature end-plate potentials (MEPPs) were not different in the Prx-/- compared with the control values in terms of amplitude and frequency (Figure 2). However, EPP latency increased significantly with age (Figure 2A). As shown in figure 2B, EPP latency in wild type mice decreased over a period of 2 months (from the second to the fourth months of age). This is probably related to the maturation of axons and myelin sheaths. In the periaxin null mutant mice, however, latency increased over the same 2 month period, reflecting the fact that Prx-/- Schwann cells initially myelinate axons normally, but this is followed by derangement of the sheath.
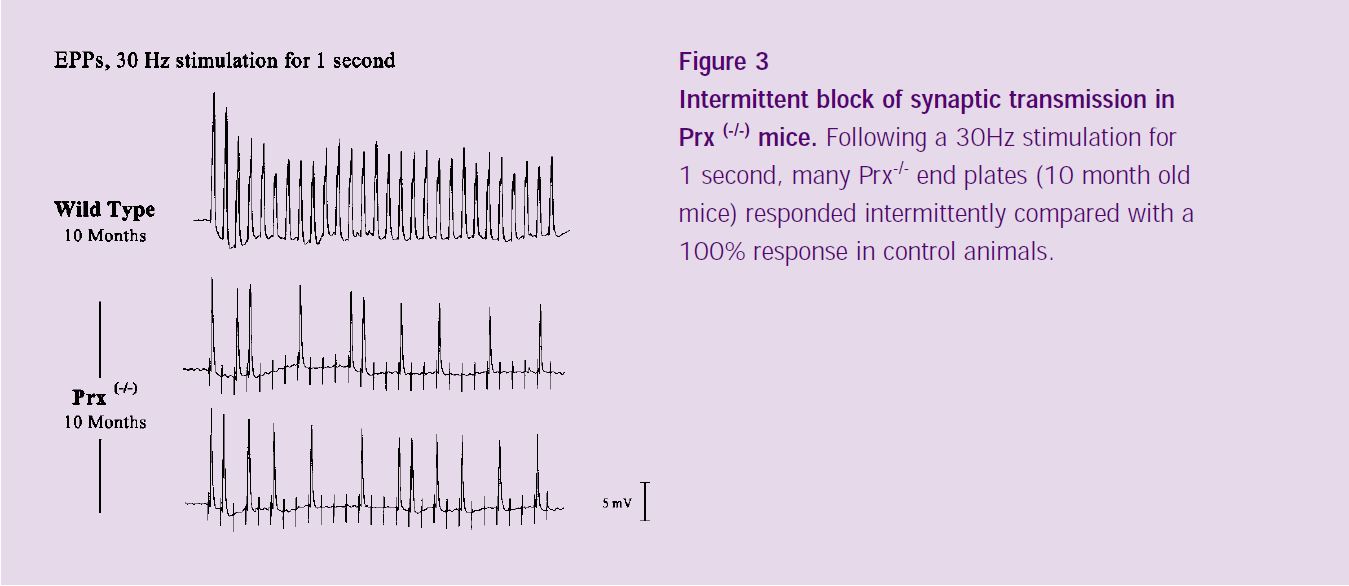
When stimulated at higher frequencies (30 Hz; Figure 3), 40% of Prx-/- endplates responded intermittently, contrasting with the complete response exhibited in wild type animals. Our preliminary immunocytochemical data (not shown) suggest that the intermittent failure of synaptic transmission in the Prx-/- mice is associated with structural abnormalities in the neuromuscular junction, consistent with branch- point failure of nerve conduction.
We are currently performing electrophysiological recording from identified neuromuscular junction using vital staining together with acetylcholine receptor staining (using rhodamine- conjugated α-bungarotoxin). We are using the vital stain FM1-43 to visualise nerve terminals by staining the recycling synaptic vesicles. This dye also stains the preterminal axon passively. Using this approach, we are attempting to correlate the morphology of the axon and postsynaptic end-plate with electrophysiological recordings from the correspondent muscle fibres (*4).
In conclusion, our observations suggest that demyelinating conditions, in addition to the known effect in proximal axons and Schwann cells, produce functional and structural changes at neuromuscular synapses.
The data obtained thus far are consistent with the hypothesis that intermittent synaptic transmission, due to branch point failure, may explain several aspects of the physiological abnormalities that accompany demyelination, including the phenotypic signs of muscle weakness and tremor.
I am supported by a Wellcome Trust Prize Studentship. I thank my supervisors, Prof. Peter Brophy and Dr. Richard Ribchester for their advice and helpful comments on this article and Mr. Derek Thomson for technical assistance with my electrophysiological recordings. I am very grateful to the Society and to Pfizer for the award of a Pfizer Prize for my presentation of some of the work described in this article at the Bristol Meeting of the Society, September 2001.
References
1: Court, FA, Brophy, PJ, & Ribchester, RR, (2001) Effects of peripheral demyelination on neuro-muscular transmission and glial cell organisation in periaxin gene-deficient mice. J Physiol Proceedings 536P, 118P.
2: Sherman, DL, Fabrizi, C, Gillespie, CS & Brophy, PJ (2001) Specific disruption of a schwann cell dystrophin-related protein complex in a demyelinating neuropathy. Neuron 30, 677-87.
3: Gillespie, CS, Sherman, DL, Fleetwood-Walker, SM, Cottrell, DF, Tait, S, Garry, EM, Wallace, VC, Ure, J, Griffiths, IR, Smith, A & Brophy, PJ (2000) Peripheral demyelination and neuropathic pain behaviour in periaxin-deficient mice. Neuron 26, 523-31.
4: Costanzo, EM, Barry, JA & Ribchester, RR (1999) Co-regulation of synaptic efficacy at stable polyneuronally innervated neuromuscular junctions in reinnervated rat muscle. J Physiol 521 Pt2, 365-74.