
Physiology News Magazine
Influence of early developmental events on breathing at birth
Features
Influence of early developmental events on breathing at birth
Features
Gilles Fortin and Jean Champagnat
Neurobiologie Génétique et Intégrative Institut de Neurobiologie Alfred Fessard CNRS, Gif-sur-Yvette, France Gilles.Fortin@iaf.cnrs-gif.fr
https://doi.org/10.36866/pn.48.9


Respiration is a rhythmic motor behaviour that appears in the fetus and acquires a vital importance at birth. It is generated centrally, within neuronal networks of the brainstem. Recently, examination of hindbrain activities in the embryo revealed that a central rhythm generator is active in the brainstem before fetal maturation and conforms to the segmented organisation of the embryonic hindbrain at this stage of development. From physiological studies of this primordial rhythm generator in embryos, we may therefore gain an understanding of how genes govern development of neuronal networks and specify patterns of motor activities operating throughout life.
Rhythm generation in the embryo
The brainstem derives from the embryonic hindbrain (rhombencephalon), one of the vesicles that appear towards the anterior end of the neural tube. When reticular neurons differentiate, the hindbrain neuroepithelium is partitioned along the antero-posterior axis into an iterated series of eight cellular compartments called rhombomeres. This segmentation process is transient and believed to specify neuronal fates by encoding positional information (Lumsden and Krumlauf, 1996). Electrophysiological recordings performed on an isolated preparation of chick embryo hindbrain revealed that by the end of the segmentation period, the hindbrain neuronal network starts to exhibit a consistent and organised activity in the form of recurring episodes composed of burst discharges that occur simultaneously in the different cranial nerves. At this stage the neuronal network is already organised with distinct reticular and motor neurons (Fortin et al, 1994). When intersegmental relationships are interrupted by transverse sectioning of the hindbrain rostral and caudal to the exit of the branchiomotor nerve, the ability to generate the rhythmic pattern is preserved in each transverse slice. The network organisation responsible for this rhythm generation conforms to the rhombomeric pattern in the hindbrain of the embryo. Specification of the future rhythmic network would therefore take place within the segmented hindbrain of the embryo and segmentation may be a determinant in conferring the ability to generate specific rhythmic patterns of activity (Champagnat et Fortin, 1997).
Identification of a maturation step leading to an High Frequency episodic activity in the chick
By recording neural rhythm generation in post-segmental chick hindbrain preparations isolated in vitro, we have identified a developmental step in which GABAergic synapses begin to exert an inhibitory action on neurons, thereby shifting rhythmic activity from low frequency (immature, figure 1, top right) to high episodic frequency (HF, closer to mature, figure 1 bottom right). At the cellular level, GABAergic inhibition is responsible for the appearance of a novel neuronal phenotype (being inhibited instead of excited during burst activity) within the rhythmic network. At the membrane level, inhibition involves GABA-A receptors and an increase of neuronal input conductance; HF results from a post-inhibitory rebound regulated by the hyperpolarisation-activated mixed cationic current, Ih (Fortin et al 1999).
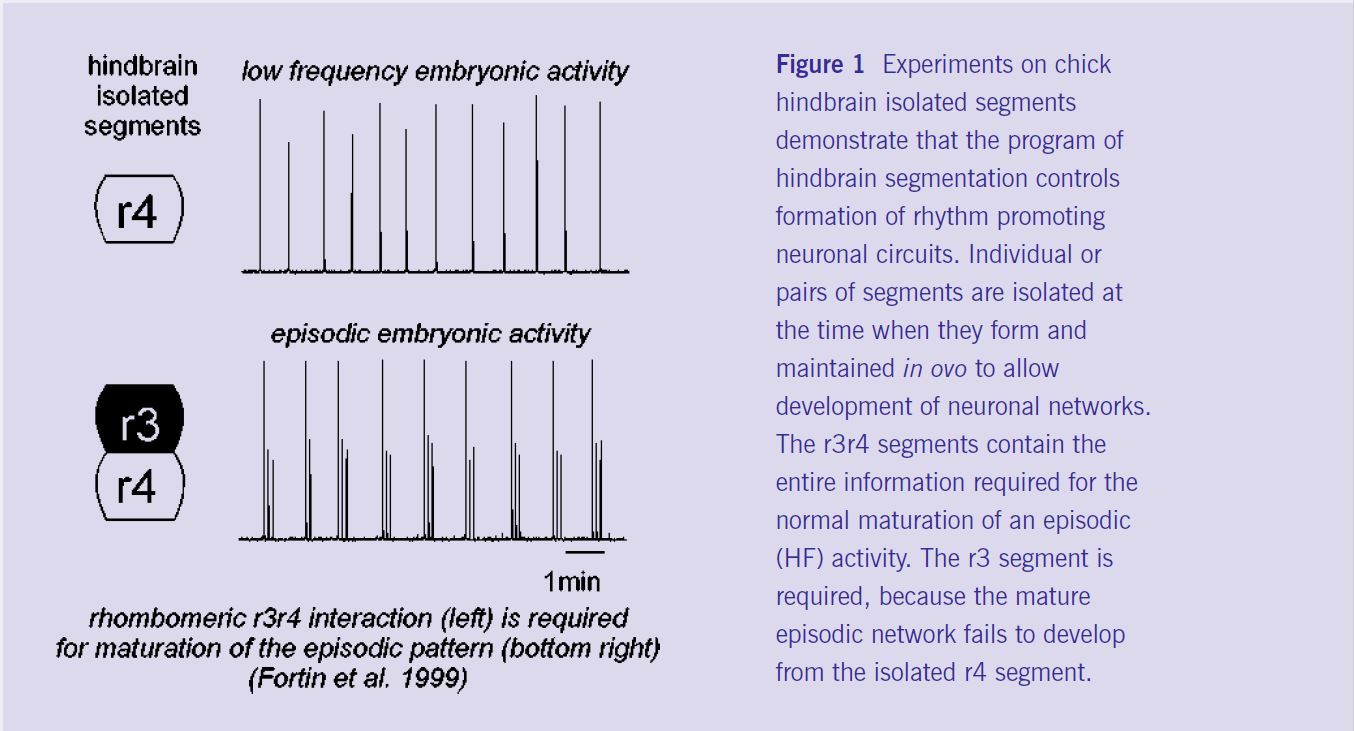
Deciphering the rhombomeric code for HF maturation.
To understand how rhombomere identity might influence rhythm generation, we have developed in collaboration with A Lumsden and S Jungbluth (London), an in ovo neural tube ablation procedure that allows the later recording of rhythms (arising after the end of the segmental period) from combinations of isolated single rhombomeres (r) or doublets of adjacent rhombomeres. We found that the normal HF generator develops in r3r4 (figure 1 bottom) and r5r6 doublets, but not in isolated r2, r4 (figure 1 top) or r6, or in r1r4, r2r3 or r4r5 doublets. Therefore, turning on the HF rhythm pattern depends on the singular identity of r3 and r5, which controls, on the basis of a two segment repeat, the later maturation of GABAergic inhibition (Fortin et al 1999). These experiments have demonstrated a robust link between rhombomere patterning and maturation of HF reticular circuits in the avian embryo.
Breathing in transgenic mice
A wealth of data has been accumulated on genes governing hindbrain segmentation. Before and at the onset of segmentation, genes encoding transcription factors such as Hox, Krox-20 and kreisler, are expressed in domains corresponding to the limits of future rhombomeres (Lumsden and Krumlauf, 1996). Inactivation of these genes specifically disturbs the rhombomeric pattern of the hindbrain. For example, Krox-20, is transiently expressed within the yet unsegmented hindbrain in two stripes with sharp edges corresponding to the future rhombomeres r3 and r5. The inactivation of Krox-20 in transgenic mice results in the deletion of r3 and r5 and lethality shortly after birth (Schneider-Maunoury et al, 1993). The Krox-20 gene product acts as a direct transcription activator of other r3- and r5-related genes belonging to the Hox clusters. Concerning Hox genes themselves, expression of Hoxa1 also provides one of the earliest signs of regionalisation within the developing hindbrain. As early as 7.5 day-post- coitum in mice, the Hoxa1 expression domain extends from the posterior end of the mouse embryo up to the presumptive r3/r4 border and is down regulated before rhombomere boundary formation. This transient expression has a profound impact on hindbrain patterning, as Hoxa1 targeted inactivation results in severe reduction of r4 and r5 and their derived structures (e.g. the motor nucleus of the facial nerve) and in lethality shortly after birth (see Mark et al, 1993). The advent of such mutant mice in which embryonic hindbrain development is affected by the deletion of specific territories provides a potential strategy to establish a link between gene expression and breathing in intact animals. Because respiration acquires a vital importance at birth, prenatal dysfunction of neuronal networks can be responsible for functional anomalies that appear after birth.
Identification of an anti-apneic neuronal system depending on the integrity of r3 or r4 in mice.
The general strategy has been to identify phenotypic traits of the rhythmic respiratory network following loss-of- function mutation of transcription factors expressed in a rhombomere- specific manner: after the elimination of both r3 and r5, or of r5 alone, obtained respectively by inactivating Krox-20 and kreisler (Chatonnet et al 2002) and after elimination of r4 and r5 in Hoxa1-/- mutants (Dominguez et al, 2001). By comparing these different mutants, it was found that both r3- and r4-derived neurons are essential to alleviate life threatening neonatal apneas thereby maintaining normal breathing shortly after birth. This “anti-apneic” system is vital during a precise time window (the first two days after birth in mice, see review by Fortin et al 2000), during which survival of Krox20-/- and Hoxa1-/- mutants could be improved by blocking enkephalinergic inhibition of the respiratory rhythm in vivo. These results have established the link between neuronal loss resulting from the abnormal segmentation of the neural tube and life-threatening murine syndromes with potential importance in neonatal human pathology.
Mis-specifications in the segmented hindbrain produce novel neuronal controls of breathing, active after birth.
Importantly, abnormal expression of segmentation genes does not necessarily result in cell death. The survival and neurobiological function of mis-specified cell progeny at birth has been investigated in Hoxa1-/- and kreisler+/- mutants. Dominguez et al (2001) have identified and located a novel functional neuronal circuit increasing the respiratory rhythm in Hoxa1-/- mice but not in wild-type mice; which seems to result from the acquisition of an r2-like phenotype by progenitors located in r3-r4 (figure 2). Chatonnet et al (2002) have found an exaggeration of the anti- apneic control (as defined above) indicating persistence of an abnormal control of respiration in heterozygous kreisler mice exhibiting mis-specifications of r3 cells (Manzanares et al, 2000), but with neither rhombomere elimination nor massive anatomical deficits. These results demonstrate that changes in segmental gene expression pattern underlie the acquisition of neuronal circuits regulating vital adaptive behaviours and, therefore, might be implicated in the evolution of the vertebrate brainstem network.
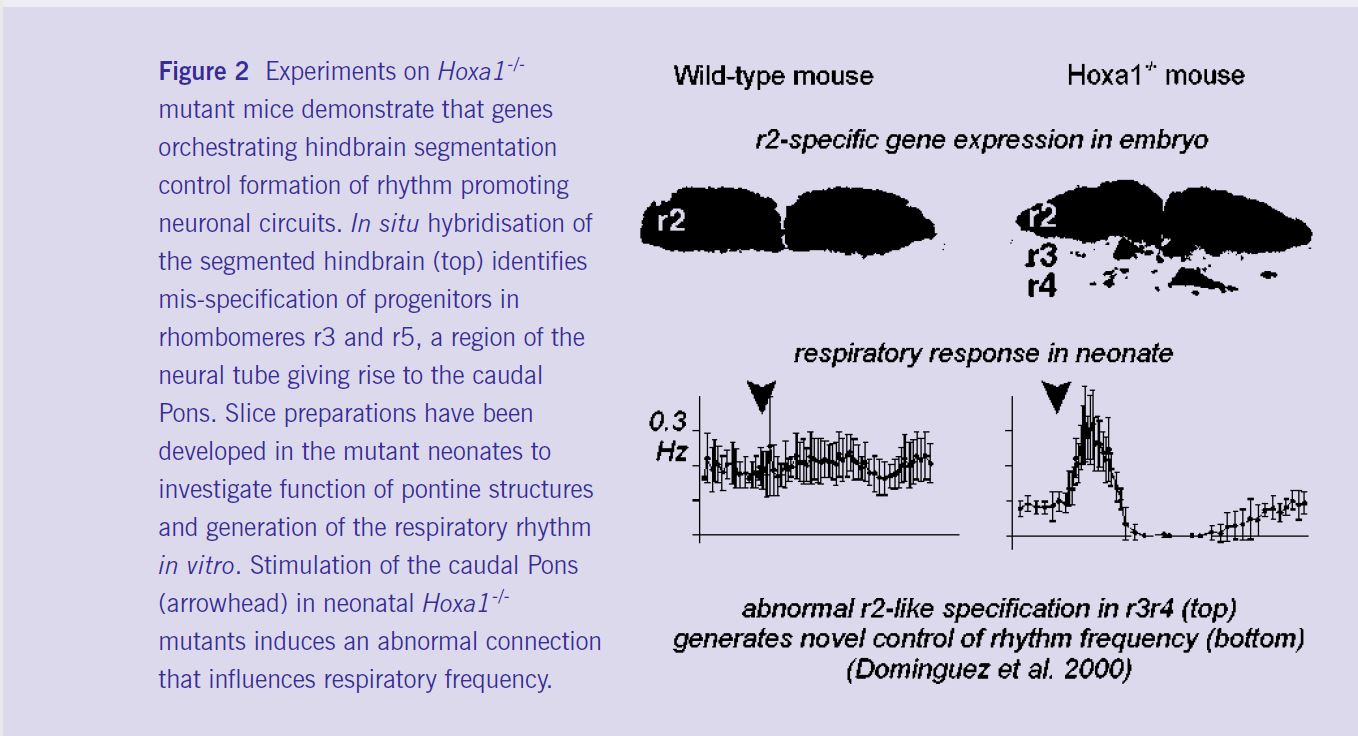
Overall, studies on embryos and neonates demonstrate the role of hindbrain segmentation in the formation of rhythm-promoting circuits. Developmental mechanisms orchestrating the early organogenesis of the brainstem appear to be crucial in establishing the postnatal breathing pattern. Experiments performed after birth in transgenic mice indicate that, although expression of these genes and hindbrain segmentation are transient events of the early embryonic development, they are important for the process of respiratory rhythm generation. Therefore, early developmental processes have to be taken into account to understand normal and pathological diversity of the vertebrate breathing behaviours during postnatal life.
Acknowledgements
Our work is supported by CNRS, INSERM, HFSP RG101/97, ACI “Development and Physiology”, EEC QLRT-2000-01467.
References
Champagnat, J and Fortin, G, 1997, Trends in Neurosciences, 20: 119-124
Chatonnet, F et al, 2002, European Journal of Neuroscience, 15: 684-692.
Fortin, G, Champagnat, J and Lumsden, A, NeuroReport, 5 (1994) 1149-1152.
Fortin, G et al, 1999, Nature Neuroscience 2: 873-877.
Fortin, G et al, 2000, Respiration Physiology, 122: 247-257.
Jacquin, TD, et al, 1996, Neuron 17: 747-758.
Lumsden A and Krumlauf R, 1996, Science 274: 1109-1115.
Manzanares et al, 1999, Developmental Biology 211 (2), 220-237.
Mark et al, 1993, Development 119:319-338.
Schneider-Maunoury et al, 1993, Cell 75: 1199-1214.
