
Physiology News Magazine
Intestinal adaptation to fasting: to live at any price
Features
Intestinal adaptation to fasting: to live at any price
Features
Caroline Habold
CNRS, CEPE, Strasbourg, France
https://doi.org/10.36866/pn.61.26

Animals in their natural habitat can survive prolonged periods of fasting: up to 4 months for the emperor penguin, one to several months for pythons, and several weeks for mammals during the cold season. In the early 1980s, Goodman et al. and Le Maho et al. showed that whole body metabolism was not constant throughout a long fast, and could be divided into three differing phases: the short phase I, characterized by the exhaustion of glycogen stores; phase II, defined by the use of lipid reserves for energy expenditure; and the late phase III, marked by an increasing protein catabolism. Even if these changes are accompanied by a severe atrophy of digestive organs, animals can re-feed successfully whatever the duration of the fasting period was, and rebuild their body reserves. What are the mechanisms permitting intestinal absorption after fasting? Are some nutrients preferably absorbed rather than others? Does whole body metabolism interfere?
Commonly, expression of intestinal hexose transporters increases with the concentration of sugar in the intestinal lumen. However, an increase in glucose absorption has been observed after ‘short’ periods of fasting i.e. corresponding to phase I or II, when the amount of glucose transporters in the enterocyte membrane should be lowered. This could be explained by an increase in membrane permeability – or even by more sodium/glucose cotransporters (SGLT1) in the apical membrane – and/or by a decrease in intracellular sodium concentration triggering glucose transport via SGLT1. The increase in the density of SGLT1 in the enterocyte apical membrane has, however, not been confirmed by other studies (for review, see Ferraris & Carey, 2000). Fructose absorption via the apical transporter GLUT5 has been shown to increase after a short fast. In contrast, the basolateral transporter GLUT2 involved in hexose release to the blood stream should not be affected by caloric restriction.
In our recent study (Habold et al. 2005), we clearly showed a difference in the expression of SGLT1 according to the metabolic and hormonal state reached by fasting rats. This effect could explain the previous differing findings. As there is no difference in the amount of SGLT1 protein and mRNA between normally fed and phase II fasting rats, we observed a significant increase from these values during the longer fasting phase III. After having perfused the small intestine with a glucose solution, we observed a significant increase in glucose absorption in phase III but also a slight one in phase II fasting rats that do not show an increase in SGLT1 transporters. This last result suggested that glucose may also cross the small intestine epithelium via a paracellular route due to an increase in epithelial permeability upon fasting. Unlike SGLT1, the facilitative apical GLUT5 and basolateral GLUT2 are downregulated throughout fasting whatever the phase is.
If GLUT2 is absent from the enterocyte basolateral membrane, how can the glucose entering in the cell at the apical pole via SGLT1, be released to the blood stream during fasting? By studying intestinal gluconeogenesis, according to the availability in gluconeogenic precursors, i.e. glycerol in phase II and amino acids in phase III fasting, we measured an increase in the gene expression, protein level and activity of the glucose-6 phosphatase (Glc6Pase) during phase III. We hypothesized that this enzyme could then be involved not only in endogenous glucose production during the fast, but also in the release of glucose to the blood stream as described by Stumpel et al. (2001) in the absence of the baso-lateral transporter GLUT2. According to this study, glucose absorbed via SGLT1 is phosphorylated to glucose-6 phosphate before entering the endoplasmic reticulum where it is hydrolyzed by Glc6Pase to glucose and phosphate. Glucose then re-enters the cytosol and diffuses out of the enterocytes by a membrane traffic pathway. So, during the late phase III of fasting, the increase in SGLT1 and Glc6Pase should permit the rapid absorption of glucose even at low concentration when food is restored (Fig. 1A). Interestingly, at this stage, animals in natural conditions as well as laboratory rats show an increase in locomotor activity consistent with a ‘search for food’ behaviour.
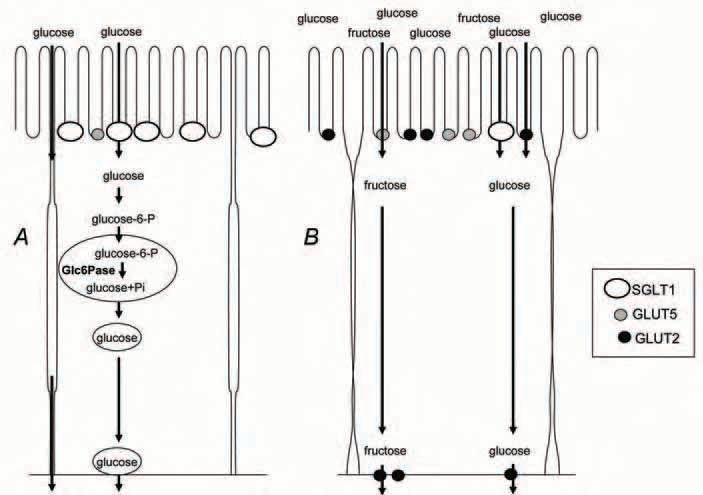
Re-feeding following either a phase II or a phase III fast stimulates the gene and protein expressions of GLUT5 and GLUT2. Furthermore, we observed that, after only 2 hours re-feeding, GLUT2 was mainly located at the apical membrane (Fig. 2). Recruitment of GLUT2 to the brush border membrane involves a protein kinase C pathway activated by glucose absorption via SGLT1 (Kellett, 2001) and can then permit absorption of glucose at high concentrations (Fig. 1B).

The unaltered and even increased absorption capabilities of the intestine during the critical phase III fast, when the animal reaches a critical threshold in nutrient reserves, coincides with a ‘search for food’ activity and could permit food assimilation immediately after re-feeding. This could thus be a survival mechanism, an ultimate energy expenditure before death in case of food becomes available.
In summary, the metabolic and hormonal status of fasted animals modifies the expression of intestinal hexose transporters, but further research is required to describe other transporters and to answer the question of whether some key nutrients may be preferentially absorbed depending on the fasting phase. This would bring new perspectives in the re-nutrition of patients suffering from severe malnutrition associated with, for example, anorexia nervosa or cancer cachexia.
References
Ferraris RP, Carey HV (2000). Intestinal transport during fasting and malnutrition. Annu Rev Nutr 20, 195-219.
Goodman MN, Larsen PR, Kaplan MN, Aoki TT, Young VR & Ruderman NB (1980). Starvation in the rat. II Effect of age and obesity on protein sparing and fuel metabolism. Am J Physiol 239, E277-E286.
Habold C, Foltzer-Jourdainne C, Le Maho Y, Lignot JH & Oudart H (2005). Intestinal gluconeogenesis and glucose transport according to body fuel availability in rats. J Physiol 566, 575-586.
Kellett GL (2001). The facilitated component of intestinal glucose absorption. J Physiol 531, 585-595.
Le Maho Y, Vu Van Kha H, Koubi H, Dewasmes G, Girard J, Ferre P & Cagnard M (1981). Body composition, energy expenditure, and plasma metabolites in long-term fasting geese. Am J Physiol 41, E342-E354.
Stumpel F, Burcelin R, Jungermann K & Thorens B (2001). Normal kinetics of intestinal glucose absorption in the absence of GLUT2: evidence for a transport pathway requiring glucose phosphorylation and transfer into the endoplasmic reticulum. Proc Natl Acad Sci USA 25, 11330-11335.
