
Physiology News Magazine
Ion channels in the ‘malaria-infected’ red blood cell
Kiaran Kirk explains how recent electrophysiological studies have provided new insights into the mechanisms by which the malaria parasite brings about a dramatic increase in the permeability of the red blood cell membrane to ions and nutrients
Features
Ion channels in the ‘malaria-infected’ red blood cell
Kiaran Kirk explains how recent electrophysiological studies have provided new insights into the mechanisms by which the malaria parasite brings about a dramatic increase in the permeability of the red blood cell membrane to ions and nutrients
Features
Kiaran Kirk
Australian National University, School of Biochemistry and Molecular Biology
https://doi.org/10.36866/pn.51.17

Some hours after the invasion of a red blood cell by the single-celled malaria parasite (Fig. 1) there are profound changes in the physiological properties of the red cell membrane. A host of small molecules have been shown to enter infected cells more rapidly than they do normal, uninfected erythrocytes and this has been attributed to the presence in the infected cell membrane of so-called ‘new permeability pathways’. These pathways are believed to serve a number of physiological roles, including the influx into the infected cell of nutrients required by the parasite, and the efflux of potentially hazardous waste products derived from the parasite’s active metabolism. The nature of these new pathways is not well understood, and their identity remains unknown. Over the last three years, however, electrophysiological studies from a number of different groups have provided new insights into the mechanisms underlying the altered membrane physiology of the parasitised red cell.

Early studies of the altered permeability of erythrocytes infected with the malaria parasite entailed the use of either radiolabelled transport substrates or measurements of the rate of haemolysis of infected cells suspended in isosmotic solutions of the substrate of interest. From these types of experiments it was evident that the pathways induced by the parasite accommodate a very wide range of low molecular weight solutes, including amino acids, sugars, nucleosides, vitamins and both inorganic and organic ions. Quantitative comparisons of influx rates showed the pathways to have a marked preference for anions over cations. Transport was shown to be non-saturable, and to be inhibited (with similar potency for a range of different transport substrates) by a range of classical ‘anion transport inhibitors’, including compounds such as furosemide niflumic acid, glibenclamide and 5-nitro-2-(3phenylpropylamino)-benzoic acid (or NPPB as it is better known). Together, the available transport data are consistent with much, if not all, of the increased flux of solutes into parasitised erythrocytes being via anion-selective (but nevertheless cation-permeable) channels of a single type.
Ion-selective channels are best studied using electrophysiological techniques. Human erythrocytes are not the easiest cells to study electrophysiologically; their small size, and their ability to squeeze through narrow openings (and to thereby disappear up the barrel of a glass microelectrode), make them difficult targets. Nevertheless, several groups have recently been successful in making electrophysiological recordings of both uninfected and malaria-infected human erythrocytes.
The first detailed characterisation of the electrophysiological characteristics of infected human erythrocyte came from Desai and colleagues, who reported that in cells infected with mature parasites the whole-cell current is 150-fold larger than that of uninfected erythrocytes (Desai et al. 2000). The increased current was attributed to the activity of a small conductance (< 10 pS) anion channel. The channel was inwardly rectifying (i.e. it passed current into the cell more readily than it did out of the cell), it was present at an estimated 1000 copies per cell, and it showed complex gating behaviour. The ion selectivity and pharmacological properties of the whole-cell currents showed close similarities to those reported previously on the basis of radiotracer flux and haemolysis experiments for the new permeability pathways induced by the parasite. Using a mathematical model to obtain quantitative permeability estimates from haemolysis experiments, the same group has shown recently that for several solutes there is good quantitative agreement between the permeabilities estimated on the basis of haemolysis experiments, radiotracer flux measurements and whole-cell current recordings (Wagner et al., 2003), consistent with the channel underlying the inwardlyrectifying current being wholly responsible for the increased transport of at least some solutes into the infected cell.
Somewhat different results were obtained in a study by Huber et al. (2002a) who, in whole-cell recordings of infected human erythrocytes, identified two discrete anion conductances, differing from one another both in their inhibitorsensitivity and in their voltagedependence. One was outwardly rectifying and the other inwardly rectifying. The conductances were diminished on treatment of the parasitised cells with reducing agents, and the same manoeuvre was shown to slow the rate of haemolysis of parasitised cells suspended in an isosmotic solution of the polyol sorbitol. In the same study it was shown that similar anion conductances could be induced in uninfected erythrocytes by exposing them to oxidizing agents, and that oxidative stress also induced haemolysis of uninfected cells suspended in an isosmotic sorbitol solution. On the basis of these observations it was postulated that the new permeability pathways induced in infected cells are endogenous erythrocyte channels, activated in response to the oxidative stress to which the host cell is subjected by the intracellular parasite. Of the two anion conductances characterized it was actually the outwardly rectifying conductance that had a pharmacological profile closest to that of the parasite-induced permeability characterised previously. A preliminary report of a differential effect of different polyols on the outwardly rectifying conductance in infected cells (Huber et al. 2002b) is also consistent with the hypothesis that the channels underlying this conductance are permeable to small organic solutes of the sort known to enter the infected cell via the parasiteinduced pathways.
The same group has reported the presence in uninfected human erythrocytes of an oxidation-induced cation conductance (Duranton et al. 2002) and have presented preliminary evidence that this conductance is activated in P. falciparum-infected cells (Tanneur et al. 2002). The conductance shows the same cation selectivity as has been reported for the transport of monovalent inorganic cations via parasiteinduced pathways (i.e. Cs+>K+>Na+>Li+; Staines et al. 2001), as well as showing an aniondependence reminiscent of the aniondependence of the uptake of both organic and inorganic cations into parasitised cells (e.g. Staines et al. 2001).
In another recent paper (Egée et al. 2002) a third group has obtained patch-clamp recordings of both uninfected and infected erythrocytes and have presented evidence for the activity in infected cells of an endogenous anion-selective channel, with a low linear conductance (~15 pS) and having properties similar, though not identical, to those of the parasite-induced channel originally described by Desai et al. (2000). Channels with the same properties could be activated in uninfected human erythrocytes either by the combination of protein kinase A and ATP, or by membrane stretch, raising the possibility that either one of these mechanisms might be involved in the activation of the channels in infected cells. In a small proportion (<5%) of excised insideout patch-clamp experiments on uninfected cells a second anion channel, showing outward rectification, was observed. But whereas Huber et al. (2002a) observed an outwardly rectifying current in a majority of infected erythrocytes and have postulated that the enhanced permeability of infected cells to small organic solutes is attributable to the channels underlying this current, Egée et al. report that the outwardly rectifying channel was “never observed in infected cell patches”. They, like Desai et al. (2000), attributed the increased conductance of the parasitised erythrocyte membrane to a single channel type.
In summary, it is clear from the spate of recent electrophysiological studies of erythrocytes infected with the malaria parasite that the membrane of the parasitized erythrocyte has a much higher electrical conductance than that of uninfected erythrocytes. The identity and number of channeltypes underlying this increased conductance is less clear. There is some evidence that the channel activity observed in infected erythrocytes is attributable to the activation of endogenous, normally quiescent, erythrocyte channels and a number of different mechanisms of channel activation have been proposed (oxidative stress, membrane stretch, protein phosphorylation). The channel originally characterized by Desai et al. (2000), and showing a close (though not exact) resemblance to that described by Egée et al. (2002) does share many characteristics with the pathways responsible for the increased permeability uptake by parasitized erythrocytes of a wide range of low molecular weight solutes. This is consistent with, though not proof of, this channel underlying the increased permeability of the infected cell. However, the relationship between this channel and the multiple conductances reported by Huber and colleagues to be active in the membrane of parasitized erythrocytes (Huber et al. 2002a; Tanneur et al. 2002) is yet to be clarified.
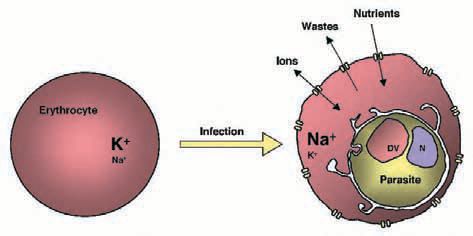
Whatever the electrophysiological characteristics and molecular identity of the pathways responsible for the increased transport rates in the parasitized red blood cell, there is growing evidence of the significance of these pathways for the intracellular parasite and its host cell (Fig. 2). At least one essential nutrient required by the parasite (the water soluble vitamin pantothenic acid) has been shown to be reliant on these pathways to gain entry into the cell (Saliba et al. 1998). Recent studies using mathematical models developed by Lew and colleagues have revealed that the leakage of Na+ and K+ (down their respective gradients) via the parasite-induced pathways, is responsible for the conversion of the erythrocyte cytosol from a high K+/low Na+ medium to a high Na+/low K+ environment for the intracellular parasite (Staines et al. 2001; Lew et al. 2003). Observations such as these highlight potentially important roles for the parasite-induced pathways in the infected cell and underscore ongoing interest in the possibility that these pathways might be suitable targets for new and much-needed antimalarial drugs.
References
Desai SA, Bezrukov SM & Zimmerberg J (2000). A voltagedependent channel involved in nutrient uptake by red blood cells infected with the malaria parasite. Nature 406:1001-1005.
Duranton C, Huber SM & Lang F (2002). Oxidation induces a Cl— dependent cation conductance in human red blood cells. J Physiol 539:847-855.
Egée S, Lapaix F, Decherf G, Staines HM, Ellory JC, Doerig C & Thomas SL (2002). A stretch-activated anion channel is upregulated by the malaria parasite Plasmodium falciparum. J Physiol 542:795-801.
Huber SM, Uhlemann AC, Gamper NL, Duranton C, Kremsner PG & Lang F (2002a). Plasmodium falciparum activates endogenous Clchannels of human erythrocytes by membrane oxidation. EMBO J 21:22-30.
Huber SM, Duranton C, Uhlemann AC, Kremsner P & Lang F 2002b). Anion and organic osmolyte channels of human erythrocytes infected with Plasmodium falciparum. Pflügers Arch 443(Supplement):S164.
Lew VL, Tiffert, T & Ginsburg, H (2003). Excess hemoglobin digestion and the osmotic stability of Plasmodium falciparum-infected red blood cells. Blood in press
Saliba KJ, Horner HA & Kirk K (1998). Transport and metabolism of the essential vitamin pantothenic acid in human erythrocytes infected with the malaria parasite Plasmodium falciparum. J Biol Chem 273:10190-10195.
Staines HM, Ellory JC & Kirk K (2001). Perturbation of the pumpleak balance for Na+ and K+ in malaria-infected erythrocytes. Am J Physiol 280:C1576-C1587.
Tanneur V, Duranton C, Lang F & Huber SM (2002). Increased cation conductance in Plasmodium falciparum-infected red blood cells. Pflügers Arch 443(Supplement):S231.
Wagner MA, Andemariam B & Desai SA (2003). A two-compartment model of osmotic lysis in Plasmodium falciparum-infected erythrocytes. Biophys J 84:116-123.
