
Physiology News Magazine
Is sensory adaptation also regulated by nuclear feedback?
Classically, sensory transduction is believed to take place solely in the cytoplasm of the cell. However, recent evidence suggests that nuclear feedback could contribute to light adaptation
Features
Is sensory adaptation also regulated by nuclear feedback?
Classically, sensory transduction is believed to take place solely in the cytoplasm of the cell. However, recent evidence suggests that nuclear feedback could contribute to light adaptation
Features
Paolo Codega, Diana E Bedolla, and Vincent Torre
Neurobiology Sector, International School for Advanced Studies (SISSA), Basovizza, Trieste, Italy
https://doi.org/10.36866/pn.76.21


For a long time, it was believed that all processes involved in sensory transduction are delimited to the cytoplasmic compartment. Receiving input such as an odour, touch or a flash of light initiates a cascade of events that codes for a signal that ultimately reaches the brain. In order to maintain efficiency, all these phenomena are fast and the sensory neurons, capable of sensing and converting the signal, must possess a ready-to-use machinery able to reach a high degree of effectiveness. Historically, sensory transduction has been viewed as an event that occurs in less than 1 s and is composed of several faster events; for this reason, scientists have mainly focused their attention on what happens at the periphery of the cell, ignoring potential nuclear involvement. If the nucleus is involved, it will regulate the level of transcription of genes encoding for enzymes and factors, influencing the signal transduction cascade. Of course, considering the time in which a nuclear feedback could happen, it is difficult to think of a role of the nucleus in sensory transduction but adaptation could be affected by nuclear feedback.

Among the different sensory systems, phototransduction is well characterised. The initial example of phototransduction was made by Wilhelm Kuhne in the 19th century, who published in the first issue of The Journal of Physiology the identification of rhodopsin, the first G-protein-coupled receptor (GPCR) (Kuhne, 1878). Among the milestones achieved in unraveling the phototransduction mechanisms were the discoveries of the light-dependent hyperpolarisation of the photoreceptor membrane (Tomita, 1970; Baylor & Hodgkin, 1973), the ‘dark current’ (Hagins et al. 1970), the first arrestin protein (Kuhn, 1978; Wilden et al. 1986), the identification of the first cyclic nucleotide-gated channel (Fesenko et al. 1985) and in 2000, the rhodopsin crystal model, which represented the first crystallographic structure of a GPCR (Palczewski et al. 2000). This sophisticated and elegantly organised biochemical pathway nowadays represents one of the best-characterised G-protein-coupled signalling pathways (Fig. 1). Phototransduction starts with the absorption of a photon that, by activating rhodopsin (R*), leads to the activation of phosphodiesterase (E*), via the G-protein transducin (G*α), causing a drop of the cytoplasmic concentration of cGMP (cG) and, ultimately, the closure of the cyclic nucleotide-gated (CNG) channels. This process is terminated by the binding of arrestin to the phosphorylated rhodopsin (R-P Arr) (Pugh & Lamb, 2000) (Fig. 1). This process takes place in the sensory neurons called photoreceptors with the final physiological outcome corresponding to a hyperpolarisation of the membrane potential caused by the inhibited ion influx (dark current) after closure of the CNG channels. A striking property of photoreceptors is their ability to operate over a vast range of light intensities that span over ten orders of magnitude, a process known as light adaptation. There are several mechanisms involved in this phenomenon, and some of them are Ca2+ dependent such as those mediated by recoverin, calmodulin and guanlylate cyclase-activating proteins (GCAPs). In general, all of the light-adaptive processes are slower than phototransduction per se and therefore, considering the difference in time scale, we hypothesised that the nucleus could come to the scene as an important component in adaptive sensory transduction.
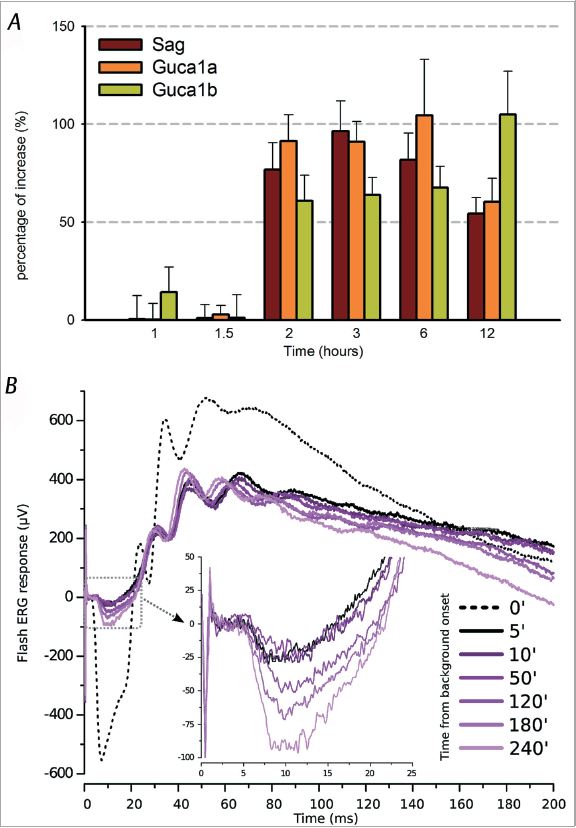
To address this issue, we screened using the DNA microarray technique on isolated light/dark-exposed photoreceptors and then confirmed with real-time PCR the up-regulation of three genes involved in phototransduction: Sag, the gene coding for arrestin, and Guca1a and Guca1b, coding for guanylate cyclase activator protein 1 and 2 (GCAPs), respectively (Codega et al. 2009). After 2 h of continuous illumination, we observed that the mRNA transcript level of these genes increases about two-fold for up to 12 h (Fig. 2A) and shows intensity-dependent behaviour. After demonstrating that this up-regulation leads to an increase of the content of their related protein, we wondered what was the physiological role of these factors. Arrestin is a potent effector for terminating phototransduction – its binding to the activated rhodopsin stops activation of the phosphodiesterase and leads to an increase of cGMP. On the other hand, GCAPs have an adaptive role: they stimulate guanylate cyclase (GC) in light (when Ca2+ level is low) in synthesising cGMP, and they inhibit GC in the dark. Therefore, an increase of arrestin and GCAPs during illumination could lead to an elevation in cytoplasmic cGMP, by increasing the efficiency of phototransduction deactivation and speeding up the GC activity. This could result in the reactivation of the dark current. To investigate this, we performed functional in vivo electroretinographic tests (ERG) in which the electrical retinal response to a bright light flash is recorded under the same light conditions used for the genomic experiments (Codega et al. 2009). The representative recording shown in Fig. 2B displays a partial recovery of the a-wave (hyperpolarising wave generated by photoreceptors); the amplitude of the a-wave was more than doubled after 240 min using background light levels that had initially almost completely suppressed the response. Intensity-dependent recoveries were always observed under the different conditions tested. These results indicate the existence of a causal relation between the observed gene up-regulation and the recovery of the photocurrent.
This work strongly suggests that the effect of light in phototransduction is not limited to events occurring in the cytoplasm, but acts also in the nucleus by regulating gene transcription. These findings open up new perspectives in the study of molecular mechanisms in sensory adaptation and, since visual and chemical transduction show great similarities in their signalling cascade, we expect that a genomic component of sensory adaptation may be present also in chemical sensory transduction and possibly also in other sensory receptors. A genomic contribution to sensory adaptation may not be confined to sensory neurons but could be present in the retina and also in the downstream elements of the perception pathways of the central nervous system. We believe that there is a place for a new player in the sensory processing and further efforts should be dedicated to unravel the role of a nuclear feedback in sensory systems.
References
Baylor DA & Hodgkin AL (1973). Detection and resolution of visual stimuli by turtle photoreceptors. J Physiol 234, 163–198.
Codega P, Santina LD, Gargini C, Bedolla DE, Subkhankulova T, Livesey FJ, Cervetto L & Torre V (2009). Prolonged illumination up-regulates arrestin and two guanylate cyclase activating proteins: a novel mechanism for light adaptation. J Physiol 587, 2457–2472. http://jp.physoc.org/content/587/11/2457.long
Fesenko EE, Kolesnikov SS & Lyubarsky AL (1985). Induction by cyclic GMP of cationic conductance in plasma membrane of retinal rod outer segment. Nature 313, 310–313.Hagins WA, Penn RD & Yoshikami S (1970). Dark current and photocurrent in retinal rods. Biophys J 10, 380–412.
Kuhn H (1978). Light-regulated binding of rhodopsin kinase and other proteins to cattle photoreceptor membranes. Biochemistry 17, 4389–4395.
Kuhne W (1878). On the stable colours of the retina. J Physiol 1, 109–212.
Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, Fox BA, Le Trong I, Teller DC, Okada T, Stenkamp RE, Yamamoto M & Miyano M (2000). Crystal structure of rhodopsin: A G protein-coupled receptor. Science, 289, 739–745.
Pugh EN & Lamb TD (2000). Phototransduction in Vertebrate Rods and Cones: Molecular Mechanisms of Amplication, Recovery and Light Adaptation. Elsevier Science.
Tomita T (1970). Electrical activity of vertebrate photoreceptors. Q Rev Biophys 3, 179–222.
Wilden U, Hall SW & Kuhn H (1986). Phosphodiesterase activation by photoexcited rhodopsin is quenched when rhodopsin is phosphorylated and binds the intrinsic 48-kDa protein of rod outer segments. Proc Natl Acad Sci U S A 83, 1174–1178.
