
Physiology News Magazine
Kidney development – consequences of disruption
Strong evidence now indicates that kidney development can be disrupted by an adverse intrauterine environment at a very early, preglomerular stage, and this may programme hypertension in the adult
Features
Kidney development – consequences of disruption
Strong evidence now indicates that kidney development can be disrupted by an adverse intrauterine environment at a very early, preglomerular stage, and this may programme hypertension in the adult
Features
E Marelyn Wintour (1), Karen Moritz (2), & Miodrag Dodic (1)
(1) Department of Physiology
(2) Department of Anatomy and Cell Biology Monash University, Australia
https://doi.org/10.36866/pn.53.14

In a recent article in The Journal of Physiology (Wintour et al. 2003) we showed that adult sheep which had developed hypertension as a result of a brief (two day) exposure to a synthetic glucocorticoid (dexamethasone) very early in development (20% of gestation) had a 40% reduction in the number of nephrons (filtering units). As nephron number is determined before birth in the sheep, as in the human, and as there was no evidence of increased destruction of nephrons in the hypertensive sheep, it was concluded that these sheep had been born with a reduced nephron number. This now seems to be quite likely as other groups have reported, recently, a reduction in nephron number in the perinatal period, in lambs which were treated with dexamethasone at mid-gestation (Figueroa et al. 2003) or subjected to undernourishment at an early stage of development (Langley-Evans et al. 2003).
There are several very interesting factors which arise from these studies. The first is that hypertension may be a consequence of perturbation of kidney development in utero, or in the period of active nephrogenesis (reviewed in Moritz et al. 2003), whereas hypertension does not necessarily result when a kidney is removed after all the nephrons have been formed. Studies in humans, rats, mice and sheep show that hypertension does result when a reduced number of nephrons occurs either by genetic influences, or by experimental removal of one kidney during the developmental period. It is assumed that when reduction in nephron number occurs during the development period, some compensatory changes occur in the remaining kidney, such as a permanent increase in the capacity to retain salt and water, and potentially changes in the expression of a number of genes, which lead to the elevation in blood pressure in the adult. These changes are not seen in the second kidney when one kidney is removed in the adult. In other words, it is not simply a reduction in nephron number which induces hypertension, but the combination of too few nephrons and some compensatory changes.
The second remarkable feature of the findings is that the ‘critical period’ in which the kidney is vulnerable to environmental factors, such as excess glucocorticoid hormones, is so early in development. In both the sheep and the rat (Wintour et al. 2003; Ortiz et al. 2002) the decrease in final number of nephrons occurred when the treatment was applied before the first nephron was fully formed. This period is early in long-gestation species (human, sheep) but comparatively late in rodents (Moritz & Wintour, 1999). As shown in the Fig. the ovine fetus, at the time of exposure to dexamethasone, had large temporary kidneys (the mesonephroi) and was at the earliest stage of the beginnings of development of the permanent kidney (metanephros). The equivalent time in human development would be 5–6 weeks (Moritz & Wintour,1999).
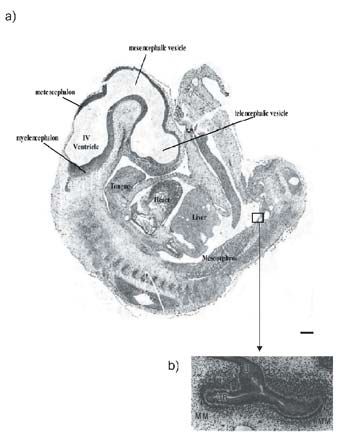
The third important implication suggested by the findings is that any factor which can disrupt nephrogenesis might also have the same consequence of programming high blood pressure in the adult offspring. There are some factors (mild vitamin A deficiency; raised blood glucose, due to type 1 or 2 diabetes which are also known to be able to reduce nephron number in the developing fetus (Moritz et al. 2003). These are far more likely to be encountered by the pregnant woman than is excess exposure to glucocorticoid at an early stage. It is estimated that there are 250 million people with mild vitamin A deficiency, of whom a substantial number are women in the childbearing years (Nelson, 2003; Christian, 2003). Whilst vitamin A treatment might not show a significant effect on parameters such as infant mortality, the long-term effects on the incidence of hypertension and renal failure have not yet been tested. There are also hundreds of millions of adults with diabetes, mostly type 2, of whom a substantial number are women in their reproductive years (Nelson, 2003). Exposure to a diabetic environment, in utero, in humans, has been shown to be associated with an increased incidence of impaired glucose tolerance, and a defective insulin secretory response in adult offspring (Sobngwi et al. 2003). The problems can thus become selfperpetuating – an unhealthy intrauterine environment leads to unhealthy mothers. The exact mechanisms by which the kidney development is so affected warrants intensive investigation.
The incidence of hypertension in people over the age of 45 years is 25%. Many of these have no genetic predisposition , or current lifestyle ‘risk factors’. For many indigenous populations (Australian Aborigines, Pima Indians, African Americans) there are high rates of renal failure (Hoy et al. 2003). It is possible that, in some cases, both hypertension and renal failure result from disrupted nephrogenesis during fetal development. Thus it is of critical importance for future health of the population to improve the health of all pregnant women.
References
Christian P (2003). Micronutrients and reproductive health issues: an international perspective. J Nutr 133, 1969S-1973S.
Hoy WE, Douglas-Denton RN, Hughson MD, Cass A, Johnson K & Bertram JF (2003). A stereological study of glomerular number and volume: preliminary findings in a multiracial study of kidneys at autopsy. Kidney Int 63, S31-S37.
Langley-Evans SC, Fahey A & Buttery PJ (2003). Early gestation is a critical period in the nutrirional programming of nephron number in sheep. Pediatr Res 53, 30A, 402.
Figueroa JP, Massmann GA, Rose JC & Zhang J (2003). Single maternal glucocorticoid administration at 80 dyas of gestation reduces fetal sheep nephron number at term. PediatrRes 53, 15A, 115.
Moritz KM & Wintour EM (1999). Functional Development of the meso- and metanephros Pediatr Nephrol 13, 171-178.
Moritz KM, Dodic M & Wintour EM (2003) Kidney Development and the fetal programming of adult disease. BioEssays 25, 212-220.
Nelson RG (2003). Intrauterine determinants of diabetic kidney disease in disadvantaged populations . Kidney Int 63,S13-S16.
Ortiz LA, Quan A, Weinberg A & Baum M (2001). Effect of prenatal dexamethasone on rat rena development. Kidney Int 59,1663-1669.
Sobngwi E, Boudou P, Mauvis-Jarvis F, Leblanc H, Velho G, Vexiau P, Porcher R, Hadjadj S, Pratley R, Tartaranni PA, Calvo F & Gautier J-F (2003). Effect of a diabetic environment in utero on predisposition to type 2 diabetes. Lancet 361, 1861-1865.
Wintour EM, Moritz KM, Johnson K, Ricardo S, Samuel CS & Dodic M (2003). Reduced nephron number in adult sheep, hypertensive as a result of prenatal glucocorticoid treatment. J Physiol 549, 929-935.
