
Physiology News Magazine
Large multiprotein complexes are involved in short-term regulation of the epithelial brush border Na+/H+ exchanger NHE3
Many transport proteins exist in large macromolecular complexes that contain scaffold proteins, signalling molecules, kinases and phosphatases. Here Mark Donowitz and colleagues describe how the Na/H antiporter NHE3 is regulated by the dynamic assembly of protein complexes
Features
Large multiprotein complexes are involved in short-term regulation of the epithelial brush border Na+/H+ exchanger NHE3
Many transport proteins exist in large macromolecular complexes that contain scaffold proteins, signalling molecules, kinases and phosphatases. Here Mark Donowitz and colleagues describe how the Na/H antiporter NHE3 is regulated by the dynamic assembly of protein complexes
Features
Mark Donowitz, Xuhang Li, & Jae Ho Kim*
Departments of Medicine and Physiology, GI Division, Johns Hopkins University School of Medicine and
*Department of Physiology, Pusan University, Pusan, Republic of Korea
https://doi.org/10.36866/pn.51.20
Our studies reflect the overlap of two themes. One of the most commonly used mechanisms for rapid regulation of transport protein function is regulated endo and exocytosis. In addition, signal transduction has been recognized to often involve large multiprotein complexes. We have described that short term regulation of the epithelial brush border Na/H exchanger NHE3 often occurs by regulated endo and exocytosis that requires NHE3 to be present in large multiprotein complexes which include the two-PDZ domain containing proteins E3KARP (NHE3 kinase A regulatory protein) or NHERF (Na/H exchange regulatory factor).
NHE3 and neutral NaCl absorption in the small intestine, in which NHE3 is linked to a brush border Cl/HCO3 exchanger, are rapidly regulated as part of digestive physiology with eating related stimulation and inhibition mimicked by neurohumoral agents/growth factors. In addition, as part of the pathobiology of diarrheal diseases, neutral NaCl absorption and NHE3 are inhibited by intestinal secretagogues. Exploration of the mechanism of this regulation has largely advanced based on studies of intact ileal mucosa and cell culture models of engineered NHE3 expressed in epithelial cells such as Caco-2 and OK and NHE null fibroblasts. In both tissue and cell culture models, regulation of NHE3 was associated with rapid changes in the amount of plasma membrane NHE3 either by stimulation of endocytosis or exocytosis, with variable additional contribution of changes in NHE3 turnover number. For instance, in ileal Na absorptive cells, stimulation of NHE3 by a2 adrenergic agonists or growth factors (EGF) was associated with an increase in the percent of NHE3 in the brush border, while inhibition of NHE3 by the cholinergic agonist carbachol was associated with a decrease in plasma membrane NHE3.
That NHE3 regulation involves multiprotein complexes has been demonstrated in ileal Na absorptive cells, the Caco-2 colon cancer cell line, the OK proximal tubule cell line from opossum, and PS120 fibroblasts. In all of these cells, NHE3 exists in complexes up to 900,000 kDa, and in ileal brush border NHE3 complexes increased in size with Ca2+ elevation (carbachol). The explanation for the large size of NHE3 complexes and the changes appears to be that NHE3 exists in a multiprotein complex under basal conditions and these change by adding or subtracting signaling or regulatory molecules as part of the NHE3 trafficking that occurs under basal conditions and that which occurs as part of rapid NHE3 regulation.
Surprisingly, when NHE3 regulation was studied in NHE null fibroblasts, most second messenger regulation failed to occur. This suggested to us that some regulatory component was missing from these cells. A yeast two-hybrid/proteomics approach was used to identify one of the interacting proteins and continues as the strategy to identify proteins involved in NHE3 regulation. One such regulatory protein was identified as interacting with the NHE3 C-terminus by yeast two-hybrid screening. The protein identified was E3KARP, which was related to another cloned protein, NHERF. The importance of NHE3 binding to E3KARP was first identified for cAMP regulation of NHE3. When E3AKRP or NHERF were expressed in PS120 cells, they reconstituted cAMP inhibition of NHE3. NHERF and E3KARP are scaffold proteins which contain two PDZ domains and a C-terminal ERM binding domain. Studies largely by Yun, Lamprecht, Weinman, Shenilokar and our group have demonstrated that second messenger regulation of NHE3 requires NHE3 to be present in large multiprotein complexes in which NHE3 binds E3KARP and/or NHERF and these PDZ domain proteins also scaffold at least some of the other proteins in the multiprotein complexes. Best studied are cAMP and Ca2+ regulation of NHE3. For cAMP regulation of NHE3, ezrin binding to E3KARP is necessary with ezrin acting as an AKAP (protein kinase A anchoring protein) for PKAII. One function of this multiprotein complex is to allow PKAII to phosphorylate NHE3.
Components of these multiprotein complexes continue to be identified, again using proteomic approaches. For Ca2+ regulation of NHE3, in addition to E3KARP, a-actinin-4 and protein kinase Ca join the complex when Ca2+ is elevated (Figs. 1-3). Actinin-4 is necessary for formation of the NHE3 complex and for NHE3 internalization, while PKCa is necessary for the complex internalization. While some basal association of PKCa with E3KARP exists, this is increased with Ca2+ elevation. While both NHERF and E3KARP reconstitute cAMP regulation, only E3KARP can reconstitute Ca2+ regulation.
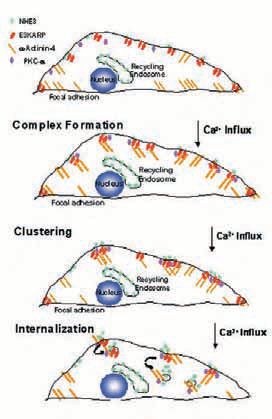
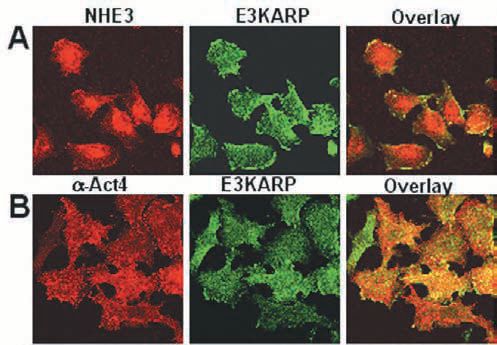
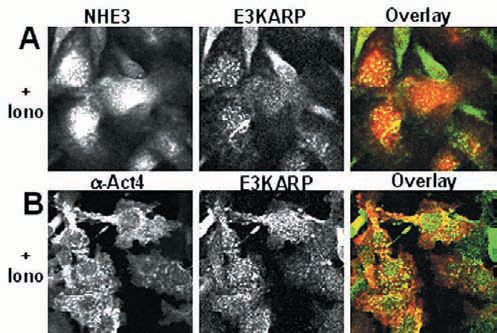
The mechanism of specificity of E3KARP in NHE3 regulation has been identified in several cases to be due to its binding of proteins involved in NHE3 regulation and for bringing them into the NHE3 complex. For instance, for Ca2+ regulation of NHE3, this results from E3KARP binding to a-actinin-4.
What is the role in NHE3 regulation of these multiprotein complexes? For cAMP and Ca2+ inhibition of NHE3, the complexes are involved in decreasing the surface amount of NHE3. The mechanism of changes in surface NHE3 involve changes in trafficking. For instance, cAMP stimulates endocytosis and inhibits exocytosis. In addition, cAMP decreases the NHE3 half-life. Thus PDZ domain protein related NHE3 complexes regulate NHE3 trafficking, with demonstrated changes in both endo and exocytosis.
Current understanding has painted a general picture of PDZ domain proteins being involved in regulation of NHE3 by taking part in formation of large NHE3-containing complexes which change as part of some signal transduction regulation of NHE3 and involve mechanisms that alter rates of NHE3 trafficking, as well as affecting NHE3 turnover number. However, this area is just being defined with many questions outstanding. These include: 1) What are the full compliment of proteins in the NHE3 complexes? 2) What is the nature of the changes in these complexes which occur with signal transduction? 3) What is the role of each component of these complexes in NHE3 regulation? 4) What aspects of NHE3 handling are affected by each complex component and by the signaling pathways activated (changes in endocytosis, exocytosis, half-life, distribution to lysosomes or proteosomes vs recycling); 5) Which of the complex components, if any, traffick with NHE3?
