Physiology News Magazine
Leptin receptor regulation – links to obesity?
Obesity is characterised by high circulating levels of leptin, a hormone that limits the amount of body fat. This paradox has led to the concept of leptin insensitivity in obesity. We investigated the regulation of leptin receptor gene expression and protein number in response to leptin and diet to see whether receptor regulation could play a role in the development of leptin insensitivity
Features
Leptin receptor regulation – links to obesity?
Obesity is characterised by high circulating levels of leptin, a hormone that limits the amount of body fat. This paradox has led to the concept of leptin insensitivity in obesity. We investigated the regulation of leptin receptor gene expression and protein number in response to leptin and diet to see whether receptor regulation could play a role in the development of leptin insensitivity
Features
Lynda M Williams (1) and Ruben Nogueiras (2)
1: Obesity and Metabolic Health Division, Rowett Institute of Nutrition and Health, University of Aberdeen, Aberdeen AB21 9SB, UK
2: Department of Physiology, School of Medicine – Instituto de Investigacion Sanitaria (IDIS), University of Santiago de Compostela and CIBER Fisiopatología de la Obesidad y Nutrición (CIBERobn), Santiago de Compostela (A Coruña), 15782, Spain
https://doi.org/10.36866/pn.78.14


Leptin was first discovered when the obesity seen in the ob/ob mutant mouse was identified as being due to a single gene mutation resulting in the complete lack of the hormone leptin. Similar mutations, although very rare, have been found in humans, with leptin replacement reversing obesity, emphasising the role of leptin in the maintenance of a lean body mass.
Leptin is mainly produced by fat. Leptin can act as a long-term signal to the brain that adequate energy is stored, and can also act as a short-term signal, with levels dropping during fasting. Leptin receptors are present in many tissues. The key site of action for leptin is in the brain, particularly the ‘energy balance centres’ in the hypothalamus. Normally, leptin signals the hypothalamus to inhibit feeding and increase energy expenditure, thereby maintaining ‘normal body weight’. However, high levels of circulating leptin, which occur in obesity, fail to stop overconsumption, leading to the concept that in obesity the loss of potency of leptin is due to leptin ‘insensitivity’. A number of different mechanisms have been proposed to account for the development of leptin insensitivity. It has been suggested that leptin receptor signalling may be impaired, for example, by high levels of suppressor of cytokine signalling 3 (SOCS3), an inhibitor of leptin signalling via the Janus kinase 2 (JAK2)/signal transducer and activator of transcription 3 (STAT3) JAK/STAT pathway. Signalling is terminated in part by the induction of SOCS3 production. The induction of SOCS3 gene expression can be used as a marker of induction of the JAK/STAT pathway.
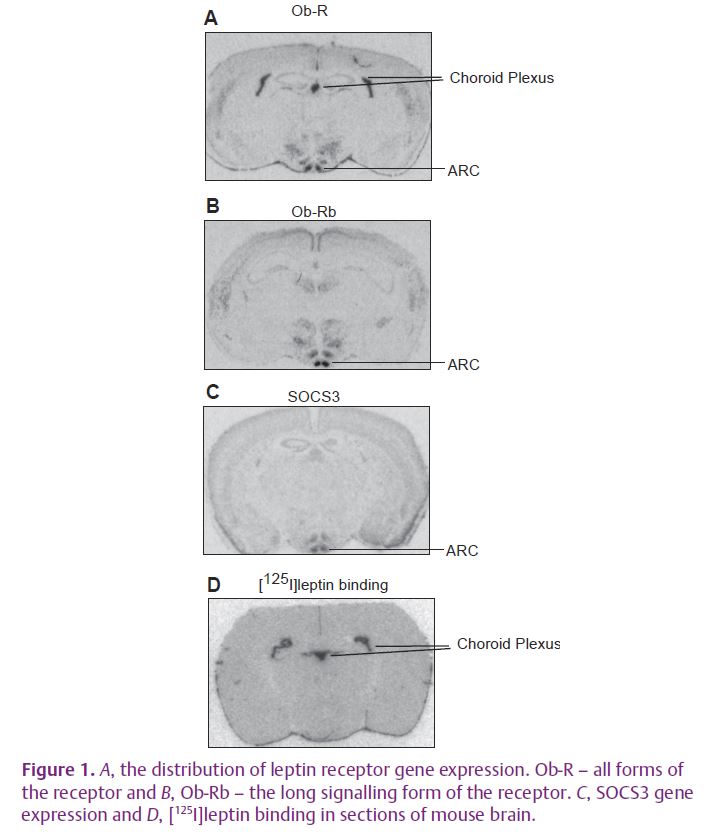
The potency of hormones is regulated in part via the presence of specific receptors. Five isoforms of the leptin receptor have been identified to date; Ob-R(a–e). However, only the long form of the receptor, Ob-Rb, is capable of eliciting a full signalling response. The short forms of the receptor, Ob-Ra and Ob-Rc, are thought to act as leptin transporters into the brain as they are present on the choroid plexus and on the brain microvessels.
We set out to look more closely at the regulation of leptin receptors in the brain, in particular the short forms on the choroid plexus and the long form on hypothalamic neurons, to find out more about how gene expression and/or receptor number were changed by circulating levels of leptin, diet and nutritional status (Mitchell et al. 2009). We used in situ hybridisation for gene expression studies and in vitro autoradiography to visualise labelling the receptor with the ligand [125I]leptin. These are ideal techniques for looking at gene expression and receptor number in complex tissues such as the brain as the signal over each anatomically distinct area can be quantified separately (Fig. 1A–D). We wanted to know what factors regulate leptin receptor gene expression and number, and whether receptor regulation could play a role in the development of leptin insensitivity.
We looked at receptor gene expression and receptor number in the brain of the C57Bl/6 mouse by manipulating the level of leptin by fasting, which drops leptin levels, refeeding, which raises leptin levels back to normal, and feeding a high-fat diet, which raises leptin levels in response to increased adiposity. We also injected leptin to further raise levels, in normal mice and those on a high-fat diet, to see if a high-fat diet would interact with the effect of leptin on the regulation of receptor gene expression or number.
A drop in the level of circulating leptin, seen in fasting, gives rise to increases in both receptor number and receptor gene expression in all regions of the brain measured. The fasting-induced drop in leptin appeared to be important for the subsequent down-regulation of gene expression and receptor number in response to increased leptin levels seen on refeeding. This was emphasised by the fact that increasing the levels of circulating leptin by both a high-fat diet and by injecting leptin had more complex effects on gene expression and receptor number depending on the region of the brain examined and on whether the long or short forms of the receptor were measured.
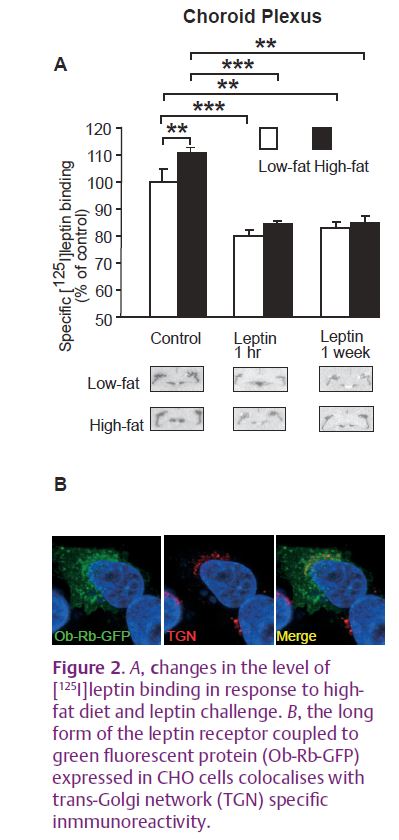
We were able to identify two potential mechanisms by which obesity may induce leptin insensitivity via receptor regulation. The first was the down-regulation of receptor number by high levels of circulating leptin (Fig. 2A). Data from studies in cells transfected with a green fluorescent protein (GFP)- tagged leptin receptor from our lab and others (Belouzard et al. 2004) shows that the endocytosis of the receptor into the cell is constitutive and that leptin has no influence over the rate of uptake into the cell. This indicates that down-regulation of receptor number at the cell surface must be the result of intracellular retention of the receptor. Leptin receptors are mainly localised to the Golgi and trans-Golgi network (TGN) in the cell (Fig. 2B). However, both smaller increases in the level of leptin induced by high-fat feeding and decreases in leptin levels during fasting, resulted in an increase in receptor number on the choroid plexus. High-fat feeding and fasting have been shown previously to down-regulate the transport of leptin into the brain (Kastin & Akerstrom, 2000; Banks et al. 2004). One explanation for this counter-intuitive increase in receptor number may be that normal transport of leptin through the cell via the receptor is inhibited by high-fat feeding, which leads to an increase in the number of receptors trapped at the cell surface.
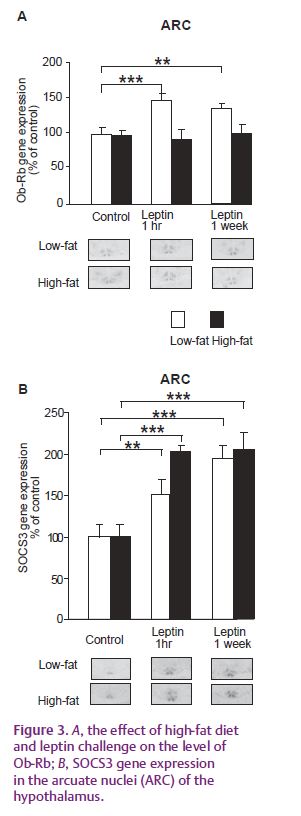
Another mechanism by which obesity may cause leptin insensitivity is the inhibition of the up-regulation of the expression of the long signalling form of the receptor by leptin (Fig. 3A). The up-regulation of the receptor by leptin was unexpected as some reports have found that a down-regulation of the receptor occurs in obesity but other studies have found no down-regulation. The increase in receptor gene expression seen in the present study is prevented by the high-fat diet but is not associated with the inhibition of leptin signalling via the JAK/STAT pathway as the induction of SOCS3 is not inhibited (Fig. 3B). This indicates that one of the other leptin signalling pathways may be important in this response – but this remains to be tested.
Our study points to the fact that the leptin receptor has evolved to be regulated by low circulating levels of leptin, whereas high circulating levels of leptin and a high-fat diet may interfere with normal regulation of gene expression and receptor number.
References
Banks WA, Coon AB, Robinson SM, Moinuddin A, Shultz JM, Nakaoke R & Morley JE (2004). Triglycerides induce leptin resistance at the blood-brain barrier. Diabetes 53, 1253–1260.
Belouzard S, Delcroix D & Rouille Y (2004). Low levels of expression of leptin receptor at the cell surface result from constitutive endocytosis and intracellular retention in the biosynthetic pathway. J Biol Chem 279, 28499–28508.
Kastin AJ & Akerstrom V (2000). Fasting, but not adrenalectomy, reduces transport of leptin into the brain. Peptides 21, 679–682.
Mitchell SE, Nogueriras R, Morris A, Tovar S, Grant C, Cruickshank M, Rayner DV, Dieguez C & Williams LM (2009). Leptin receptor gene expression and number in the brain are regulated by leptin level and nutritional status. J Physiol 587, 3573–3585. http://jp.physoc.org/content/587/14/3573.full