
Physiology News Magazine
Light up the signal fires – imaging calcium’s protein partners
Features
Light up the signal fires – imaging calcium’s protein partners
Features
Stephen Bolsover
Department of Physiology, University College London, UK
https://doi.org/10.36866/pn.62.37

In Victorian times, almost all microscopes sold came with an attachment to facilitate the observation of blood flow in the tail of a tadpole. Using this, the paterfamilias could demonstrate to his family the opaque red blood corpuscles coursing through otherwise invisible vessels that branched and anastomized. More advanced microscopists killed, fixed and stained the tadpole to reveal the various tissues in more or less detail.
Ten years ago our study of calcium signalling in cells was at a similar stage. Thanks to the indicator dyes created by Roger Tsien and others we could see where calcium ions accumulated, and the paths through which they moved through the cytoplasm. By killing and fixing the cell we could see its organelles and even the location of individual proteins – at the time of fixation – in exquisite detail. However, we could not watch the interaction between calcium and other components in a living cell. The development of green fluorescent protein and other novel live cell imaging technologies has changed this situation dramatically. We can now see where particular proteins or organelles are in living cells, and watch how this pattern overlaps with, or changes in response to, calcium signals. The most advanced protein constructs go further and report not only their location but also their conformation.

The immediate targets of calcium are the calcium binding proteins, among which calmodulin dominates. Figure 1 shows two ways in which calcium and calmodulin can interact spatially. In nerve cell bodies calmodulin is distributed relatively uniformly (panel A) while electrical activity evokes a calcium signal that is largest at the cell periphery (B). In contrast, stimulation of mast cells releases calcium from intracellular stores causing a relatively uniform calcium increase (E), but calmodulin is concentrated at the periphery (D). Stimulation of both types of cell generates a high concentration of active calmodulin at the periphery, as revealed by imaging of a reporter calmodulin (C).
Because the calcium transient and the spatial distribution of the target protein have to overlap for a response to be elicited, the system constitutes an ‘and’ gate. For example, the neurotransmitter glutamate will cause the calcium concentration to increase in the nuclei of hippocampal neurones, but this calcium increase can only activate the nuclear transcription factor CREB if calmodulin has previously been caused to move to the nucleus by electrical activity (Mermelstein et al. 2001).
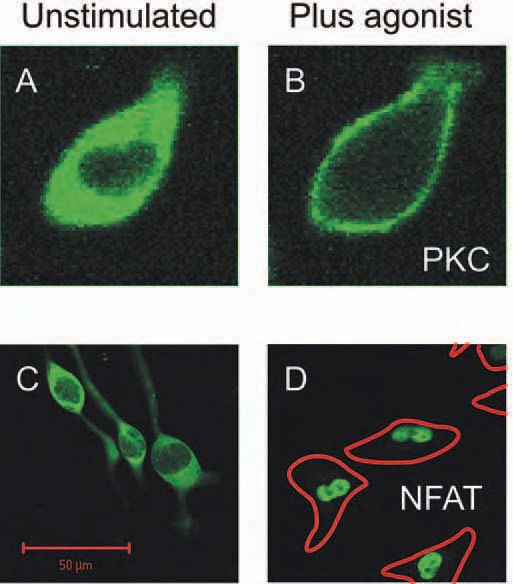
The movement of calmodulin to the nucleus of electrically active neurons is one example of calcium-evoked translocation. The mechanisms that cause proteins to move in response to a calcium signal are many, and vary in complexity. Some proteins use calcium ions as part of the coupling that attaches them to a cellular location. A rise of calcium concentration in that cell region will therefore recruit the protein. Examples are protein kinase C (Fig. 2A, B) (Bolsover et al. 2003) and annexin. Other proteins are posttranslationally modified in response to a calcium signal. Their subsequent movement need not, therefore, be dependent on where the calcium signal occurred. The transcription factor NFAT behaves in this way (Fig. 2C, D). It bears a nuclear localization signal that is only active when the protein is dephosphorylated by the calmodulin dependent phosphatase calcineurin (PP2B). Thus NFAT can be dephosphorylated at the location where calcium increases (assuming that calcineurin is present); its subsequent movement to the nucleus will be calcium-independent. Since the time constant of NFAT rephosphorylation is of the order of 7 minutes (Tomida et al. 2003), even low frequency calcium transients can cause a maintained NFAT translocation (Pandey et al. 2004).
With the help of GFP and other fluorescent labels, achieving our goal of mapping the interactions of calcium and its downstream targets in living cells is literally in sight.
References
Bolsover SR, Gomez-Fernandez JC & Corbalan-Garcia S (2003). Role of the Ca2+/phosphatidylserine-binding region of the C2 domain in the translocation of protein kinase Calpha to the plasma membrane. J Biol Chem 278, 10282 – 10290.
Mermelstein PG, Deisseroth K, Dasgupta N, Isaksen AL & Tsien RW (2001). Calmodulin priming: nuclear translocation of a calmodulin complex and the memory of prior neuronal activity. Proc Natl Acad Sci USA 98, 15342-15347.
Milikan JM & Bolsover SR (2000). Use of fluorescently labelled calmodulins as tools to measure subcellular calmodulin activation in living dorsal root ganglion cells. Pflügers Archiv 439, 394-400.
Milikan JM, Carter TD, Horne JH, Tzortzopoulos A, Török K & Bolsover SR (2002). Integration of calcium signals by calmodulin in rat sensory neurones. Eur J Neurosci 15, 661-670.
Pandey V, Mihara S, Fensome-Green A, Bolsover SR & Cockcroft S (2004). Monomeric IgE stimulates NFAT translocation into the nucleus, a rise in cytosol Ca2+, degranulation and membrane ruffling in the cultured rat basophilic leukemia-2H3 mast cell-line. J Immunol 172, 4048-4058.
Tomida T, Hirose, K, Takizawa A, Shibasaki F & Iino M (2003). NFAT functions as a working memory of Ca2+ signals in decoding Ca2+ oscillation. EMBO J 22, 3825-3832.
