
Physiology News Magazine
Long-lasting cellular imprinting
Performance hacking towards the Olympics?
Features
Long-lasting cellular imprinting
Performance hacking towards the Olympics?
Features
Dr Einar Eftestøl, University of Oslo, Norway
Professor Jo Christiansen Bruusgaard, Kristiania University College, Norway
https://doi.org/10.36866/122.34
Muscle memory is a term that for decades has been associated with the ability to learn specific motor tasks. Repeated practice enables complex muscle contractions like playing the piano or riding a bicycle to be performed more or less automatically, but the term is not very precise, as it really involves changes that occur in the brain, and not in the muscle cells. However, during the last decade several findings indicate that the muscle cells themselves have the ability to “remember” former greatness.
A cellular muscle memory
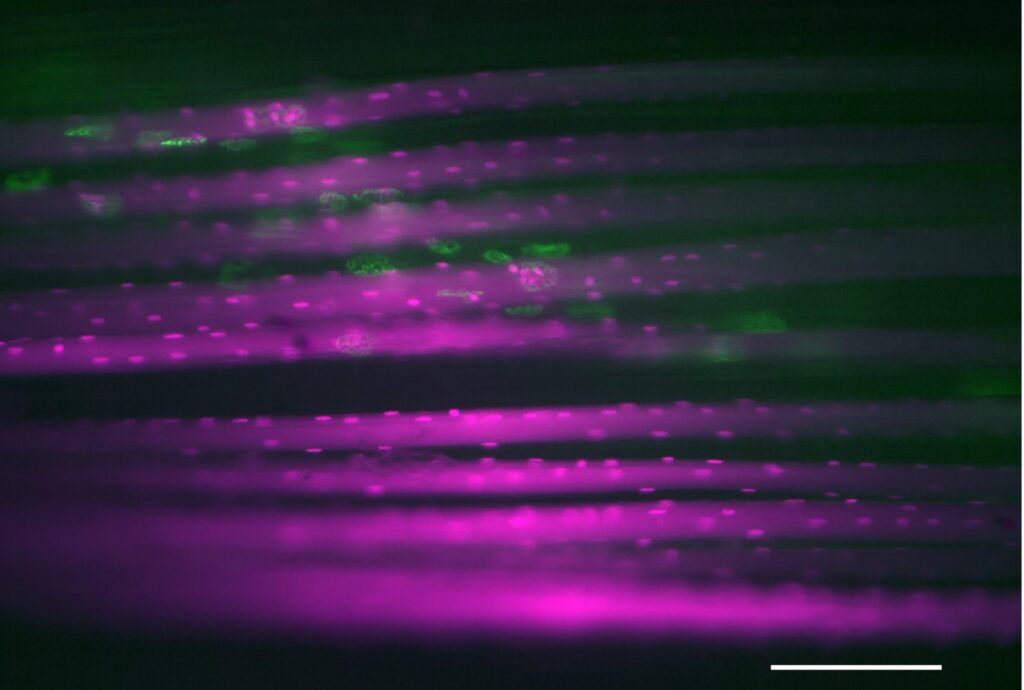
Muscle cells are unique in several aspects compared with other cells, but most prominent is their immense size and the fact that they contain hundreds if not thousands of nuclei (Fig. 1). More than 10 years ago we, together with several other researchers in our group, found that when subjecting rodent muscle to mechanical overload, the addition of new nuclei to the muscle cells preceded muscle growth, and remained in the cells even after severe muscle atrophy (Bruusgaard et al., 2010). Furthermore, muscle growth induced by anabolic steroids in mice added more nuclei in a similar manner, and after a long period without exposure to anabolic steroids these muscles appeared to have a significant growth advantage when subjected to mechanical overload (Egner et al., 2013) (Fig. 2). We described this phenomenon as a cellular form of muscle memory residing in the muscle cells themselves, with the number of muscle fibre nuclei (or myonuclei) as a “memory storage unit”. The hypothesis that the number of myonuclei represents an advantage for muscle growth has generated some controversy (McCarthy et al., 2017), although the absolute need for myonuclei in order for muscle fibres to complete their postnatal developmental growth has recently been verified by transgenic mice that leave satellite cells fusion-incompetent. By inducing the transgene at different developmental timepoints, satellite cell fusion can be stopped and the number of myonuclei specifically titrated (Cramer et al., 2020).
Epigenetics and cellular memory
Research during the last decade has also investigated the phenomenon of a cellular memory in lieu of epigenetic alterations. Epigenetics is defined as non-sequence structural modifications of DNA and/ or histones that alter patterns of gene expression, and diverse environmental stimuli can provoke epigenetic responses in many different cell types that can last for decades, and even be transferred to offspring, one example being maternal nutritional status (Vineis et al., 2017). Current evidence also supports a long-lasting exercise-induced epigenetic memory in skeletal muscle (Beiter et al., 2020), whereby previous strength training, leading to long-lasting changes in DNA methylation patterns, aided future muscle mass gains in humans. Thus, all environmental stimuli that can lead to short- term cellular adaptation can lead to epigenetic imprinting that bear the potential of a long- term cellular memory. It even provides a possible epigenetic explanation for the idea of athletes breeding athletes.

Performance-enhancing drugs
Based on the current knowledge about cellular muscle memory, together with evidence for an epigenetic memory in response to a number of physiological stimuli in different cell types, establishment of a cellular memory after abuse of performance-enhancing drugs is also likely. As environmental stimuli can lead to epigenetic memory in haematopoietic stem cells (makers of red blood cells) (Vineis et al., 2017), a similar epigenetic memory response to erythropoietin abuse seems plausible. For steroids it has been established that testosterone has the potential to induce epigenetic programming in mice (Dkhil et al., 2015), and that strength exercise induces epigenetic muscle memory in humans. In our opinion this makes it plausible that testosterone can lead to an epigenetic memory in skeletal muscle.
Long-lasting effects in humans
Do performance-enhancing drugs give a competitive edge in a long-term perspective for elite athletes? Indeed, anabolic steroids induce both increases in strength (Bhasin et al., 1996) and myonuclear number (Kadi et al., 1999), but few studies have been able to investigate this in a controlled manner. However, a hint towards long-lasting effects appears in the doping programme of the German Democratic Republic government, starting in the mid-60s. Here, the first documented case of androgenic doping of a woman is described by Franke et al.: “after 4 years of systematic androgenization, her basic strength level even when not taking the drug had also increased” (Franke and Berendonk, 1997). It should be noted that some of this retention of strength might be a result of the muscle’s ability to keep much of its gained size (Psilander et al., 2019), and not in a muscle memory phenomenon per se, but this would still represent a long-lasting performance effect.
Hacking your performance
With recent findings on muscle memory as the backdrop, it is timely to debate whether early use of performance-enhancing drugs can result in a permanent advantage in exercise adaptability, giving that extra edge needed to outperform others in elite sports. If advantages from the intake of performance- enhancing drugs can be present long after its traceability, it leads to dystopic consequences for elite sports: one can use performance- enhancing drugs before even being considered for an anti-doping programme, involving minimal risk of getting caught, and stand on top of the podium in the Olympics much later. In elite sports, the margins are often small, and a 2% improvement in performance can be the difference between winning and coming last in a 100 m race.
Considering this, even a small residual effect of former doping, whether it resides in an epigenetic imprint, increased number of nuclei or retained muscle mass, the result is the same: former doping could lead to an advantage even long after the doping took place. In light of these observations, even longer exclusion periods should be considered, and novel methods with the potential to detect previous doping use should be explored.
References
Beiter T et al. (2020). Transcriptional memory in skeletal muscle. Don’t forget (to) exercise. Journal of Cellular Physiology 235, (7-8), 5476–5489. https://doi.org/10.1002/jcp.29535
Bhasin S et al. (1996). The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men. New England Journal of Medicine 335, 1–7. https://doi.org/110.1056/NEJM199607043350101
Bruusgaard, JC et al. (2010). Myonuclei acquired by overload exercise precede hypertrophy and are not lost on detraining. Proceedings of the National Academy of Sciences of the United States of America 107(34), 15111–15116. https://doi.org/10.1073/pnas.0913935107
Cramer AAW et al. (2020). Nuclear numbers in syncytial muscle fibers promote size but limit the development of larger myonuclear domains. Nature Communications 11, 6287. https://doi.org/10.1038/s41467-020-20058-7
Dkhil MA et al. (2015). Epigenetic modifications of gene promoter DNA in the liver of adult female mice masculinized by testosterone. The Journal of Steroid Biochemistry and Molecular Biology 145, 121–130. https://doi.org/10.1016/j.jsbmb.2014.11.006
Egner IM et al. (2013). A cellular memory mechanism aids overload hypertrophy in muscle long after an episodic exposure to anabolic steroids. The Journal of Physiology 591(24), 6221–6230. https://doi.org/10.1113/jphysiol.2013.264457
Franke WW and Berendonk B (1997). Hormonal doping and androgenization of athletes: a secret program of the German Democratic Republic government. Clinical Chemistry 43(7), 1262–1279. https://doi.org/10.1093/clinchem/43.7.1262
Kadi F et al. (1999). Effects of anabolic steroids on the muscle cells of strength-trained athletes. Medicine & Science in Sports & Exercise 31(11), 1528–1534. https://doi.org/10.1097/00005768-199911000-00006
McCarthy JJ et al. (2017). Methodological issues limit interpretation of negative effects of satellite cell depletion on adult muscle hypertrophy. Development 144(8), 1363–1365. https://doi.org/10.1242/ dev.145797
Psilander N et al. (2019). Effects of training, detraining, and retraining on strength, hypertrophy, and myonuclear number in human skeletal muscle. Journal of Applied Physiology 126(6), 1636–1645. https://doi.org/10.1152/japplphysiol.00917.2018
Vineis P et al. (2017). Epigenetic memory in response to environmental stressors. FASEB J 31(6), 2241–2251. https://doi.org/10.1096/fj.201601059RR
World Anti-Doping Agency (2015). World Anti-Doping Code [Online]. Available: https://www.wada-ama.org/sites/default/files/resources/files/wada-2015-world-anti-doping-code.pdf [Accessed 11 May 2021].
