
Physiology News Magazine
Lymphatic vessels – absorptive sumps or leaky pumps?
Lymphatic vessels, the absorptive vessels of the body, are responsible for catching fluid filtered from capillaries and returning it to the bloodstream, a process necessary for life. A commonly espoused hypothesis for their success is that the lymphatic vessel wall is impermeable to solute that has already entered from the tissue through the initial lymphatics. Contrary to this textbook view, recent studies demonstrate that lymphatic vessels are surprisingly leaky.
Features
Lymphatic vessels – absorptive sumps or leaky pumps?
Lymphatic vessels, the absorptive vessels of the body, are responsible for catching fluid filtered from capillaries and returning it to the bloodstream, a process necessary for life. A commonly espoused hypothesis for their success is that the lymphatic vessel wall is impermeable to solute that has already entered from the tissue through the initial lymphatics. Contrary to this textbook view, recent studies demonstrate that lymphatic vessels are surprisingly leaky.
Features
Joshua P Scallan and Virginia H Huxley
Department of Medical Pharmacology & Physiology, University of Missouri School of Medicine, Columbia, MO, USA
https://doi.org/10.36866/pn.80.16

All blood microvessels leak proteins, sugars, gases and water into the tissues, be they arterioles, capillaries or venules. By virtue of their resemblance to these blood vessels, shouldn’t lymphatic vessels leak solute and water as well? In fact, this thought process led to studies examining protein loss from the lymphatic vasculature by Mayerson et al. (1962), when they infused canine leg lymphatics with radiolabelled albumin and collected lymph simultaneously from the thoracic duct (where the lymphatic vasculature empties into the blood). As Fig.1 depicts, Mayerson et al. detected less than 3% of the infused albumin in blood; from this they concluded that the lymphatic circulation was virtually impermeable to albumin. Probably as a consequence, over the next half-century many assumed that these vessels were impermeable to protein, otherwise their contents would leak right back out into the tissue thereby negating their primary function.
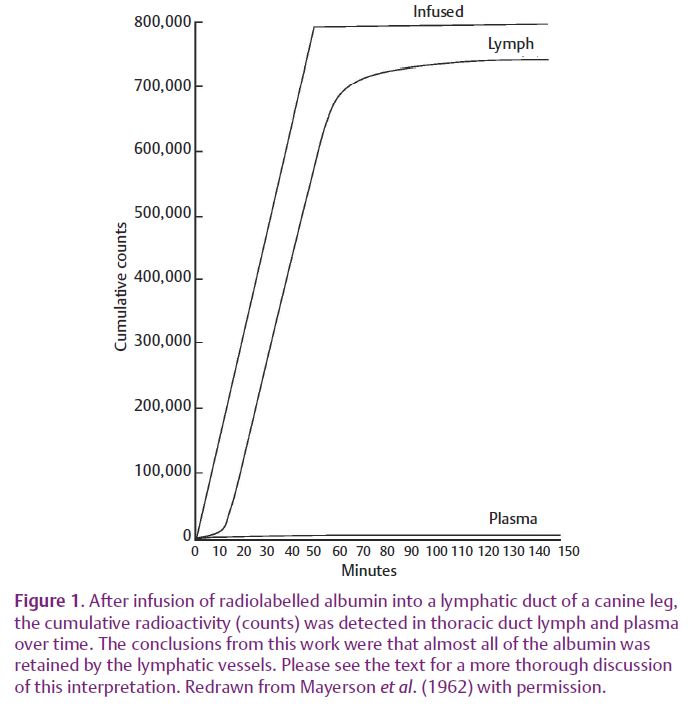
Less emphasized, however, were their studies demonstrating that smaller molecules crossed the lymphatic wall so rapidly that they equilibrated with the tissues and blood. In this work, the rate at which molecules were lost across the lymphatic vessel wall decreased as the molecular size increased, a phenotype discovered earlier for blood microvessels (Mayerson et al. 1962).
Unfortunately, measuring the total amount of protein crossing lymphatic vessels per se is not very informative because it does not quantify the permeability of a vessel to a molecule. ‘Permeability’ is a coefficient describing the ease with which molecules traverse a vessel wall, and is more accurate because it is a measure of the amount of solute that moves across a vessel segment over time (flux) when both its surface area and the concentration gradient are known (and unchanging). Accounting for such variables may seem trivial at first, but increasing the vessel surface area or concentration gradient will increase the flux of molecules moving across the lymphatic wall, making the vessel appear falsely as more or less ‘permeable’. Discussing solute movement in terms of permeability also enables direct comparison of data from vessels separated spatially or temporally.
A newer technique borrowed from those studying blood microvessels, where fluorescently labelled proteins are perfused through vessel segments isolated from tissue, facilitates the measurement of lymphatic vessel permeability. While the data are sparse, the dependence of solute movement on molecular size has been confirmed for the lymphatics (Ono et al. 2005; Price et al. 2008). The latter study (Price et al. 2008), in which lymphatic endothelial cells (LECs) were grown inside artificial tubes made of collagen, reported absolute permeability values that, for comparison, were approximately 10 times that measured for venules (Scallan & Huxley, 2010). Whether it is justifiable to make conclusions of in vivo lymphatic solute transport from an artificial system lacking several barrier components is debatable.
One group to date has assessed lymphatic permeability in vivo by cannulation and perfusion with fluorescently labelled albumin (Scallan & Huxley, 2010) to test the hypothesis that lymphatic vessels containing valves and spontaneously contracting muscle cells (called collecting lymphatics) possess a basal permeability to albumin no different from the venules. Since LECs are derived from cardinal vein endothelium during development (Srinivasan et al. 2007), their rationale was that LECs share many genes, and probably functions, with blood endothelium. The study concluded that collecting lymphatic permeability to albumin did not differ from that of venules (Fig. 2). Furthermore, the permeability of initial lymphatics – devoid of valves and muscle cells – was determined to be similar to the LEC tubes with an ~10-fold greater permeability than the venules. Therefore, heterogeneity exists with respect to permeability among the different lymphatic vessel types, the same being true for blood microvessels (e.g. venules and capillaries are leakier than arterioles).
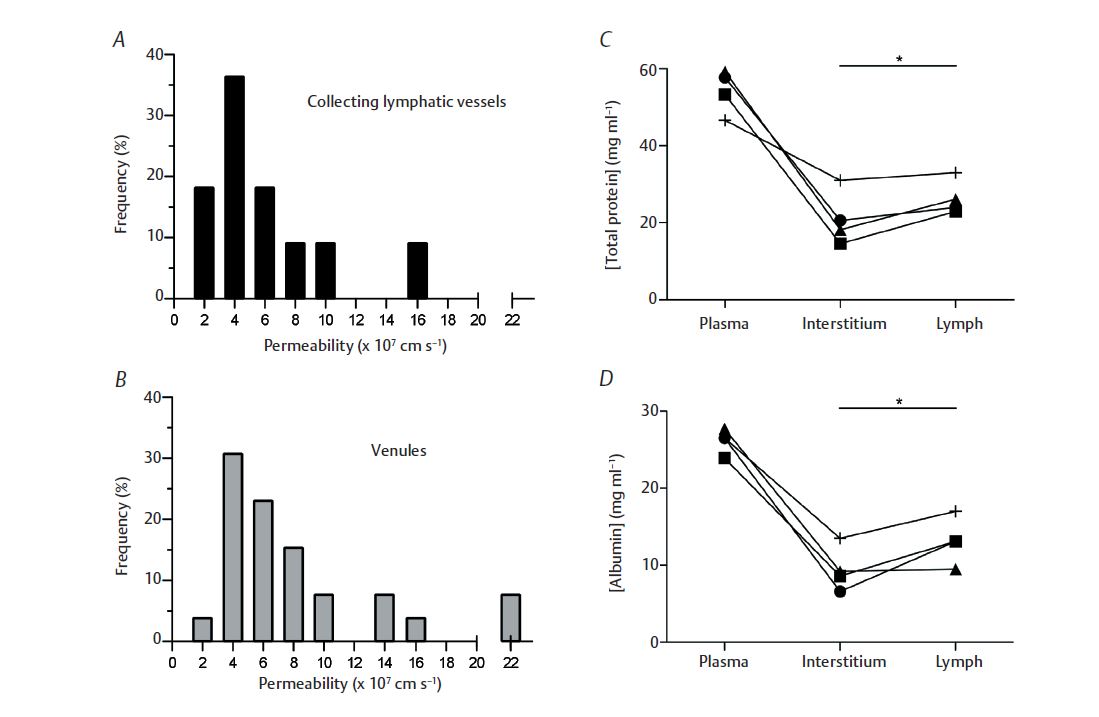
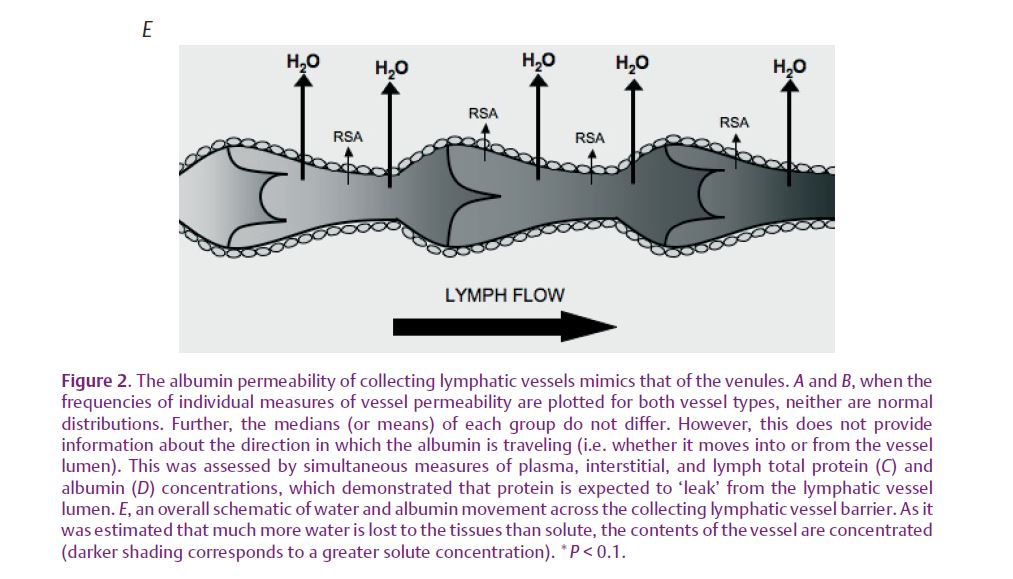
Okay, lymphatic vessels are as permeable as blood microvessels, but where does all this solute go? For vessels, the sum of hydrostatic and osmotic pressures determines whether water and solute movement is directed towards the interstitium (net leak) or towards the vessel lumen (net absorption). Pressure, as well as vessel and tissue protein concentrations, must be measured simultaneously to know these forces. Well established for blood vessels is that water is generally filtered into the interstitium, carrying with it macromolecules such as albumin, which diffuses down its concentration gradient into the tissue. However, no experiments have performed matched measurements of collecting lymphatic, tissue and blood protein concentrations. Scallan & Huxley (2010) not only made these paired measures, but also recorded vessel hydrostatic pressures prior to their experiments. Thus, the data in Fig. 2 demonstrate that collecting lymphatics possess a greater protein concentration in their lumen than in the tissue, favouring diffusion of albumin out of the vessel. Additionally, collecting lymphatics all maintained hydrostatic pressures well into the positive range, so that one may expect net fluid filtration into the tissue.
Upon reconsideration of their main function – to absorb fluid and protein for return to the blood – these conclusions seem contradictory. How can lymphatic vessels both absorb and leak protein-containing fluid? Examining the two lymphatic vessel types provides clues. Initial lymphatics form the entryway into this vasculature; consequently their hydrostatic and osmotic driving forces are low. Collecting lymphatics are larger, downstream contractile vessels possessing greater pressures and driving forces. Therefore, protein absorbed by the initial lymphatics will enter the collecting lymphatics where only a percentage will diffuse into the tissues down a concentration gradient. Reinforcing this conclusion Scallan & Huxley
(2010) estimated that: (1) the collecting lymphatics filter more water than protein, effectively concentrating luminal protein and steepening the concentration gradient and (2) solute will be entrained by the filtering water (see Fig. 2).
The field investigating microvessel permeability is small, such that our complete knowledge of lymphatic vessel permeability has been presented here. Undoubtedly, much remains to be learned about this second circulatory system, but what can be gleaned from the aforementioned experiments demonstrates that the lymphatic vasculature is capable of providing nutrients to tissues in regions where blood vessels are scarce while at the same time regulating fluid homeostasis.
References
Mayerson HS, Patterson RM, McKee A, LeBrie SJ & Mayerson P (1962). Permeability of lymphatic vessels. Am J Physiol 203, 98–106.
Ono N, Mizuno R & Ohhashi T (2005). Effective permeability of hydrophilic substances through walls of lymph vessels: roles of endothelial barrier. Am J Physiol Heart Circ Physiol 289, H1676–H1682.
Price GM, Chrobak KM & Tien J (2008). Effect of cyclic AMP on barrier function of human lymphatic microvascular tubes. Microvasc Res 76, 46–51.
Scallan JP & Huxley VH (2010). In vivo determination of collecting lymphatic vessel permeability to albumin: a role for lymphatics in exchange. J Physiol 588, 243–254. http://jp.physoc.org/content/588/1/243.long
Srinivasan RS, Dillard ME, Lagutin OV, Lin FJ, Tsai S, Tsai MJ, Samokhalov IM & Oliver G (2007). Lineage tracing demonstrates the venous origin of the mammalian lymphatic vasculature. Genes Dev 21, 2422–2432.
